Downregulation of Sp1 by Minnelide leads to decrease in HSP70 and decrease in tumor burden of gastric cancer
- PMID: 28192510
- PMCID: PMC5305197
- DOI: 10.1371/journal.pone.0171827
Downregulation of Sp1 by Minnelide leads to decrease in HSP70 and decrease in tumor burden of gastric cancer
Abstract
Background: Gastric cancer is the third leading cause of cancer related mortality worldwide with poor survival rates. Even though a number of chemotherapeutic compounds have been used against this disease, stomach cancer has not been particularly sensitive to these drugs. In this study we have evaluated the effect of triptolide, a naturally derived diterpene triepoxide and its water soluble pro-drug Minnelide on several gastric adenocarcinoma cell lines both as monotherapy and in combination with CPT-11.
Methods: Gastric cancer cell lines MKN28 and MKN45 were treated with varying doses of triptolide in vitro. Cell viability was measured using MTT based assay kit. Apoptotic cell death was assayed by measuring caspase activity. Effect of the triptolide pro-drug, Minnelide, was evaluated by implanting the gastric cancer cells subcutaneously in athymic nude mice.
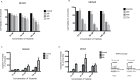
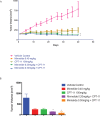
Results: Gastric cancer cell lines MKN28 and MKN45 cells exhibited decreased cell viability and increased apoptosis when treated with varying doses of triptolide in vitro. When implanted in athymic nude mice, treatment with Minnelide reduced tumor burden in both MKN28 derived tumors as well as MKN45 derived tumors. Additionally, we also evaluated Minnelide as a single agent and in combination with CPT-11 in the NCI-N87 human gastric tumor xenograft model.
Conclusion: Our results indicated that the combination of Minnelide with CPT-11 resulted in significantly smaller tumors compared to control. These studies are extremely encouraging as Minnelide is currently undergoing phase 1 clinical trials for gastrointestinal cancers.
Conflict of interest statement
University of Minnesota has a patent for Minnelide, which has been licensed to Minneamrita Therapeutics, LLC. AS is the co-founder and the Chief Scientific Officer of this company. SB is a consultant with Minneamrita Therapeutics LLC and this relationship is managed by University of Minnesota and University of Miami. DVH is the PI on the phase I clinical trial for Minnelide. This does not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

