Acquired resistance to oxaliplatin is not directly associated with increased resistance to DNA damage in SK-N-ASrOXALI4000, a newly established oxaliplatin-resistant sub-line of the neuroblastoma cell line SK-N-AS
- PMID: 28192521
- PMCID: PMC5305101
- DOI: 10.1371/journal.pone.0172140
Acquired resistance to oxaliplatin is not directly associated with increased resistance to DNA damage in SK-N-ASrOXALI4000, a newly established oxaliplatin-resistant sub-line of the neuroblastoma cell line SK-N-AS
Abstract
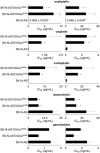
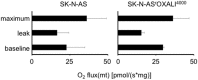
The formation of acquired drug resistance is a major reason for the failure of anti-cancer therapies after initial response. Here, we introduce a novel model of acquired oxaliplatin resistance, a sub-line of the non-MYCN-amplified neuroblastoma cell line SK-N-AS that was adapted to growth in the presence of 4000 ng/mL oxaliplatin (SK-N-ASrOXALI4000). SK-N-ASrOXALI4000 cells displayed enhanced chromosomal aberrations compared to SK-N-AS, as indicated by 24-chromosome fluorescence in situ hybridisation. Moreover, SK-N-ASrOXALI4000 cells were resistant not only to oxaliplatin but also to the two other commonly used anti-cancer platinum agents cisplatin and carboplatin. SK-N-ASrOXALI4000 cells exhibited a stable resistance phenotype that was not affected by culturing the cells for 10 weeks in the absence of oxaliplatin. Interestingly, SK-N-ASrOXALI4000 cells showed no cross resistance to gemcitabine and increased sensitivity to doxorubicin and UVC radiation, alternative treatments that like platinum drugs target DNA integrity. Notably, UVC-induced DNA damage is thought to be predominantly repaired by nucleotide excision repair and nucleotide excision repair has been described as the main oxaliplatin-induced DNA damage repair system. SK-N-ASrOXALI4000 cells were also more sensitive to lysis by influenza A virus, a candidate for oncolytic therapy, than SK-N-AS cells. In conclusion, we introduce a novel oxaliplatin resistance model. The oxaliplatin resistance mechanisms in SK-N-ASrOXALI4000 cells appear to be complex and not to directly depend on enhanced DNA repair capacity. Models of oxaliplatin resistance are of particular relevance since research on platinum drugs has so far predominantly focused on cisplatin and carboplatin.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



References
-
- Schroeder A, Heller DA, Winslow MM, Dahlman JE, Pratt GW, Langer R, et al. Treating metastatic cancer with nanotechnology. Nat Rev Cancer. 2012;12: 39–50. - PubMed
-
- Domingo-Domenech J, Vidal SJ, Rodriguez-Bravo V, Castillo-Martin M, Quinn SA, Rodriguez-Barrueco R, et al. Suppression of acquired docetaxel resistance in prostate cancer through depletion of notch- and hedgehog-dependent tumor-initiating cells. Cancer Cell 2012;22: 373–388. 10.1016/j.ccr.2012.07.016 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
- BB/E010652/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BB/E024211/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- G19438/2/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- MR/L00870X/1/MRC_/Medical Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

