The efficacy of a scaffold-free Bio 3D conduit developed from human fibroblasts on peripheral nerve regeneration in a rat sciatic nerve model
- PMID: 28192527
- PMCID: PMC5305253
- DOI: 10.1371/journal.pone.0171448
The efficacy of a scaffold-free Bio 3D conduit developed from human fibroblasts on peripheral nerve regeneration in a rat sciatic nerve model
Abstract
Background: Although autologous nerve grafting is the gold standard treatment of peripheral nerve injuries, several alternative methods have been developed, including nerve conduits that use supportive cells. However, the seeding efficacy and viability of supportive cells injected in nerve grafts remain unclear. Here, we focused on a novel completely biological, tissue-engineered, scaffold-free conduit.
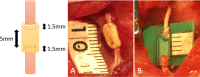
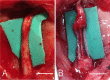
Methods: We developed six scaffold-free conduits from human normal dermal fibroblasts using a Bio 3D Printer. Twelve adult male rats with immune deficiency underwent mid-thigh-level transection of the right sciatic nerve. The resulting 5-mm nerve gap was bridged using 8-mm Bio 3D conduits (Bio 3D group, n = 6) and silicone tube (silicone group, n = 6). Several assessments were conducted to examine nerve regeneration eight weeks post-surgery.
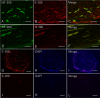
Results: Kinematic analysis revealed that the toe angle to the metatarsal bone at the final segment of the swing phase was significantly higher in the Bio 3D group than the silicone group (-35.78 ± 10.68 versus -62.48 ± 6.15, respectively; p < 0.01). Electrophysiological studies revealed significantly higher compound muscle action potential in the Bio 3D group than the silicone group (53.60 ± 26.36% versus 2.93 ± 1.84%; p < 0.01). Histological and morphological studies revealed neural cell expression in all regions of the regenerated nerves and the presence of many well-myelinated axons in the Bio 3D group. The wet muscle weight of the tibialis anterior muscle was significantly higher in the Bio 3D group than the silicone group (0.544 ± 0.063 versus 0.396 ± 0.031, respectively; p < 0.01).
Conclusions: We confirmed that scaffold-free Bio 3D conduits composed entirely of fibroblast cells promote nerve regeneration in a rat sciatic nerve model.
Conflict of interest statement
There are no patents or marketed products to declare. KN is the co-founder and shareholder of Cyfuse Biomedical K.K., Tokyo, Japan (Cyfuse). SA and MT, who are employees of Cyfuse, contributed to the manufacturing of 3D conduits and Cyfuse provided the bioprinter to manufacture the conduit. The company has the industrial rights related to the bioprinting method used to construct the 3D conduit in this work. Cyfuse provided support in the form of salaries for authors SA, KN, and MT and provided research grants to RI, TA, KN and SM. These competing interests do not alter the authors' adherence to PLOS ONE policies on sharing data and materials.
Figures




References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

