CD8+ T cell cytotoxicity mediates pathology in the skin by inflammasome activation and IL-1β production
- PMID: 28192528
- PMCID: PMC5325592
- DOI: 10.1371/journal.ppat.1006196
CD8+ T cell cytotoxicity mediates pathology in the skin by inflammasome activation and IL-1β production
Abstract
Deregulated CD8+ T cell cytotoxicity plays a central role in enhancing disease severity in several conditions. However, we have little understanding of the mechanisms by which immunopathology develops as a consequence of cytotoxicity. Using murine models of inflammation induced by the protozoan parasite leishmania, and data obtained from patients with cutaneous leishmaniasis, we uncovered a previously unrecognized role for NLRP3 inflammasome activation and IL-1β release as a detrimental consequence of CD8+ T cell-mediated cytotoxicity, ultimately resulting in chronic inflammation. Critically, pharmacological blockade of NLRP3 or IL-1β significantly ameliorated the CD8+ T cell-driven immunopathology in leishmania-infected mice. Confirming the relevance of these findings to human leishmaniasis, blockade of the NLRP3 inflammasome in skin biopsies from leishmania-infected patients prevented IL-1β release. Thus, these studies link CD8+ T cell cytotoxicity with inflammasome activation and reveal novel avenues of treatment for cutaneous leishmaniasis, as well as other of diseases where CD8+ T cell-mediated cytotoxicity induces pathology.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
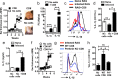
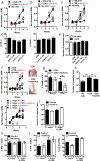
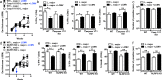
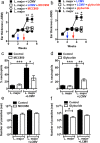

References
-
- Nitcheu J, Bonduelle O, Combadiere C, Tefit M, Seilhean D, et al. (2003) Perforin-dependent brain-infiltrating cytotoxic CD8+ T lymphocytes mediate experimental cerebral malaria pathogenesis. J Immunol 170: 2221–2228. - PubMed
-
- Silverio JC, Pereira IR, Cipitelli Mda C, Vinagre NF, Rodrigues MM, et al. (2012) CD8+ T-cells expressing interferon gamma or perforin play antagonistic roles in heart injury in experimental Trypanosoma cruzi-elicited cardiomyopathy. PLoS Pathog 8: e1002645 10.1371/journal.ppat.1002645 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

