First-line antiretroviral drug discontinuations in children
- PMID: 28192529
- PMCID: PMC5305232
- DOI: 10.1371/journal.pone.0169762
First-line antiretroviral drug discontinuations in children
Abstract
Introduction: There are a limited number of paediatric antiretroviral drug options. Characterising the long term safety and durability of different antiretrovirals in children is important to optimise management of HIV infected children and to determine the estimated need for alternative drugs in paediatric regimens. We describe first-line antiretroviral therapy (ART) durability and reasons for discontinuations in children at two South African ART programmes, where lopinavir/ritonavir has been recommended for children <3 years old since 2004, and abacavir replaced stavudine as the preferred nucleoside reverse transcriptase inhibitor in 2010.
Methods: We included children (<16 years at ART initiation) who initiated ≥3 antiretrovirals between 2004-2014 with ≥1 follow-up visit on ART. We estimated the incidence of first antiretroviral discontinuation using Kaplan-Meier analysis. We determined the reasons for antiretroviral discontinuations using competing risks analysis. We used Cox regression to identify factors associated with treatment-limiting toxicity.
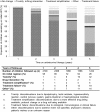
Results: We included 3579 children with median follow-up duration of 41 months (IQR 14-72). At ART initiation, median age was 44 months (IQR 13-89) and median CD4 percent was 15% (IQR 9-21%). At three and five years on ART, 72% and 26% of children respectively remained on their initial regimen. By five years on ART, the most common reasons for discontinuations were toxicity (32%), treatment failure (18%), treatment simplification (5%), drug interactions (3%), and other or unspecified reasons (18%). The incidences of treatment limiting toxicity were 50.6 (95% CI 46.2-55.4), 1.6 (0.5-4.8), 2.0 (1.2-3.3), and 1.3 (0.6-2.8) per 1000 patient years for stavudine, abacavir, efavirenz and lopinavir/ritonavir respectively.
Conclusions: While stavudine was associated with a high risk of treatment-limiting toxicity, abacavir, lopinavir/ritonavir and efavirenz were well-tolerated. This supports the World Health Organization recommendation to replace stavudine with abacavir or zidovudine in paediatric first-line ART regimens in order to improve paediatric first-line ART durability.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
-
- World Health Organization. Guideline on when to start antiretroviral therapy and on pre-exposure prophylaxis for HIV. Geneva: WHO press; 2015. - PubMed
-
- World Health Organization. Scaling up Antiretroviral Therapy in Resource-Limited Settings: Treatment Guidelines for a Public Health Approach, 2003 Revision. Geneva: WHO; 2004.
-
- World Health Organization. Antiretroviral therapy of HIV infection in infants and children: towards universal access Geneva: WHO; 2006. - PubMed
-
- World Health Organization. Antiretroviral therapy for HIV infection in infants and children: Towards universal access Recommendations for a public health approach: 2010 revision. Geneva: WHO Press; 2010. - PubMed
-
- World Health Organization. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection Recommendations for a public health approach. Geneva; WHO Press; 2013. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials