Multi-layered control of Galectin-8 mediated autophagy during adenovirus cell entry through a conserved PPxY motif in the viral capsid
- PMID: 28192531
- PMCID: PMC5325606
- DOI: 10.1371/journal.ppat.1006217
Multi-layered control of Galectin-8 mediated autophagy during adenovirus cell entry through a conserved PPxY motif in the viral capsid
Abstract
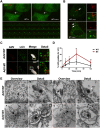
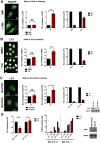
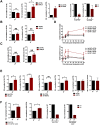
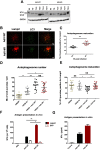
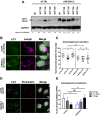
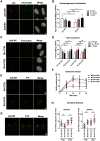
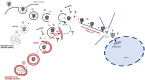
Cells employ active measures to restrict infection by pathogens, even prior to responses from the innate and humoral immune defenses. In this context selective autophagy is activated upon pathogen induced membrane rupture to sequester and deliver membrane fragments and their pathogen contents for lysosomal degradation. Adenoviruses, which breach the endosome upon entry, escape this fate by penetrating into the cytosol prior to autophagosome sequestration of the ruptured endosome. We show that virus induced membrane damage is recognized through Galectin-8 and sequesters the autophagy receptors NDP52 and p62. We further show that a conserved PPxY motif in the viral membrane lytic protein VI is critical for efficient viral evasion of autophagic sequestration after endosomal lysis. Comparing the wildtype with a PPxY-mutant virus we show that depletion of Galectin-8 or suppression of autophagy in ATG5-/- MEFs rescues infectivity of the PPxY-mutant virus while depletion of the autophagy receptors NDP52, p62 has only minor effects. Furthermore we show that wildtype viruses exploit the autophagic machinery for efficient nuclear genome delivery and control autophagosome formation via the cellular ubiquitin ligase Nedd4.2 resulting in reduced antigenic presentation. Our data thus demonstrate that a short PPxY-peptide motif in the adenoviral capsid permits multi-layered viral control of autophagic processes during entry.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
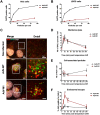
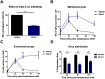
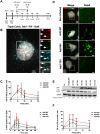
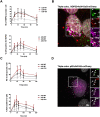
References
-
- Wickham TJ, Mathias P, Cheresh DA, Nemerow GR. Integrins αvβ3 and αvβ5 promote adenovirus internalization but not virus attachment. Cell. 1993. April 23;73(2):309–19. - PubMed
-
- Greber UF, Willetts M, Webster P, Helenius A. Stepwise dismantling of adenovirus 2 during entry into cells. Cell. 1993. November 5;75(3):477–86. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

