Generating evidence on a risk-based monitoring approach in the academic setting - lessons learned
- PMID: 28193170
- PMCID: PMC5307807
- DOI: 10.1186/s12874-017-0308-6
Generating evidence on a risk-based monitoring approach in the academic setting - lessons learned
Abstract
Background: In spite of efforts to employ risk-based strategies to increase monitoring efficiency in the academic setting, empirical evidence on their effectiveness remains sparse. This mixed-methods study aimed to evaluate the risk-based on-site monitoring approach currently followed at our academic institution.
Methods: We selected all studies monitored by the Clinical Trial Unit (CTU) according to Risk ADApted MONitoring (ADAMON) at the University Hospital Basel, Switzerland, between 01.01.2012 and 31.12.2014. We extracted study characteristics and monitoring information from the CTU Enterprise Resource Management system and from monitoring reports of all selected studies. We summarized the data descriptively. Additionally, we conducted semi-structured interviews with the three current CTU monitors.
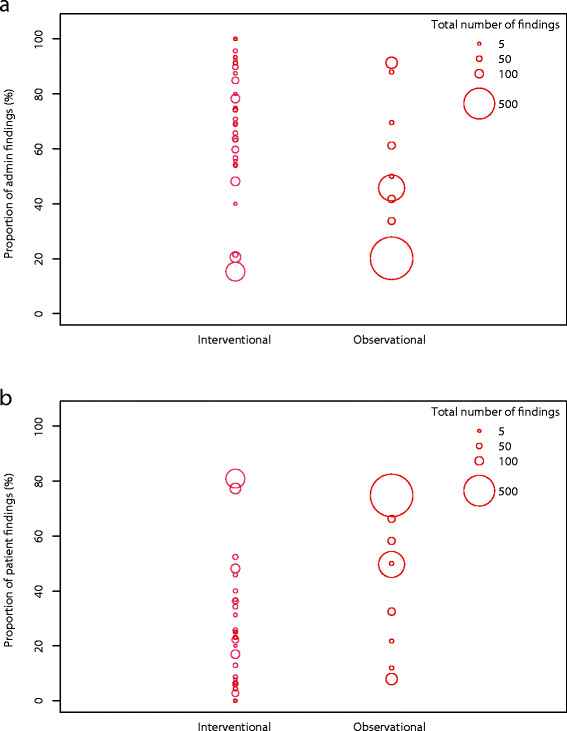
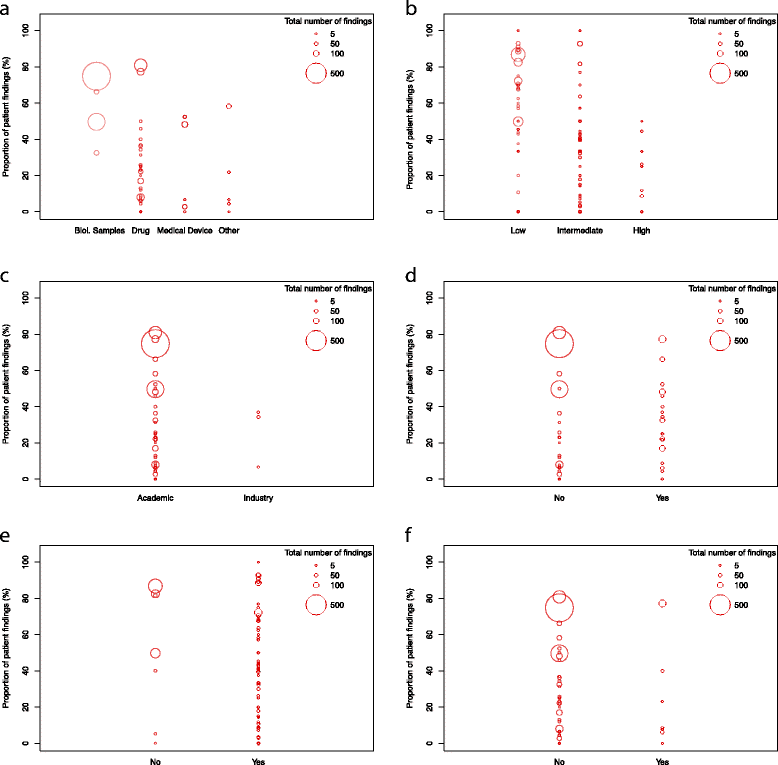
Results: During the observation period, a total of 214 monitoring visits were conducted in 43 studies resulting in 2961 documented monitoring findings. Our risk-based approach predominantly identified administrative (46.2%) and patient right findings (49.1%). We identified observational study design, high ADAMON risk category, industry sponsorship, the presence of an electronic database, experienced site staff, and inclusion of vulnerable study population to be factors associated with lower numbers of findings. The monitors understand the positive aspects of a risk-based approach but fear missing systematic errors due to the low frequency of visits.
Conclusions: We show that the factors mostly increasing the risk for on-site monitoring findings are underrepresented in the current risk analysis scheme. Our risk-based on-site approach should further be complemented by centralized data checks, allowing monitors to transform their role towards partners for overall trial quality, and success.
Keywords: Academia; Monitoring; On-site monitoring; Quality Assurance; Risk proportionate; Risk-based.
Figures

References
-
- Castle PE, Cuzick J, Stoler MH, Wright TC, Jr, Reid JL, Dockter J, Giachetti C, Getman D. Detection of human papillomavirus 16, 18, and 45 in women with ASC-US cytology and the risk of cervical precancer: results from the CLEAR HPV study. Am J Clin Pathol. 2015;143(2):160–167. doi: 10.1309/AJCPLCD8TTOMLJTB. - DOI - PubMed
-
- The International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use, ICH Harmonised Tripartite Guideline: Guideline for Good Clinical Practice. https://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Ef.... Accessed 11 Nov 2016.
-
- International conference on harmonisation of technical requirements for registration of pharmaceuticals for human use, Intergrated Addendum to ICH E6(R1): Guideline for Good Clinical Practice E6R(2). http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Eff.... Accessed 11 Nov 2016.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

