Exceptional serological and radiological response to sorafenib in 2 patients with advanced hepatocellular carcinoma and chronic hepatitis C viral infection: case report and review of the literature
- PMID: 28193171
- PMCID: PMC5307848
- DOI: 10.1186/s12876-017-0585-x
Exceptional serological and radiological response to sorafenib in 2 patients with advanced hepatocellular carcinoma and chronic hepatitis C viral infection: case report and review of the literature
Abstract
Background: In patients with advanced hepatocellular carcinoma (HCC), the multikinase inhibitor sorafenib is the only systemic treatment that has been shown to increase overall survival. However, similar to other tyrosine kinase inhibitors, most patients achieve disease stabilisation radiologically, and only 2-3% of patients achieve a partial response. Recent exploratory subgroup analyses of the large phase 3 trials have demonstrated that patients with chronic hepatitis C virus (HCV) infection associated HCC survive longer than those who are negative for HCV. The mechanism underlying this currently remains unknown. A small number of cases of complete response to sorafenib treatment have now been reported worldwide, however a prolonged response has only been reported in 2 cases, both of whom had HCV-related HCC.
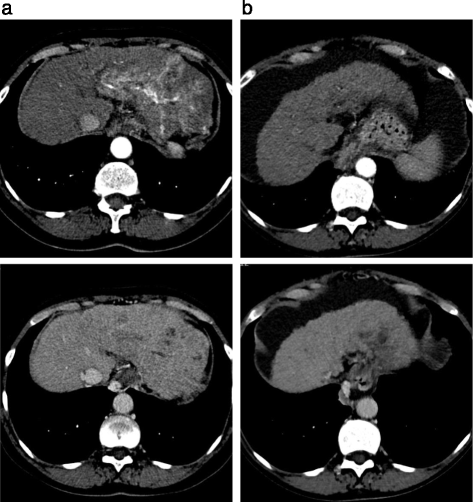
Case presentation: A 55 year old gentleman was diagnosed with hepatocellular carcinoma and concomitant chronic hepatitis C viral infection. He progressed following transarterial chemoemoblisation treatment and was commenced on sorafenib treatment. His serum alphafetoprotein level normalised within 2 months of treatment and he achieved an almost complete radiological response. This response was maintained for 20 months before the patient progressed. A 75 year old lady was diagnosed with advanced hepatocellular carcinoma and concomitant chronic hepatitis C viral infection. She was commenced on sorafenib treatment but required early dose reductions due to palmar plantar erythrodysesthesia, and liver decompensation. Despite this she achieved an excellent serological and radiological response that was maintained for 24 months.
Conclusions: Our two cases show that patients with HCV-associated HCC can attain excellent responses to sorafenib treatment that is durable. Furthermore, such exceptional responses can be achieved even with dose reductions and treatment breaks.
Keywords: Hepatitis C virus infection; Hepatocellular carcinoma; Sorafenib.
Figures

References
-
- Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25–34. doi: 10.1016/S1470-2045(08)70285-7. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

