Automated freeze-thaw cycles for decellularization of tendon tissue - a pilot study
- PMID: 28193263
- PMCID: PMC5307874
- DOI: 10.1186/s12896-017-0329-6
Automated freeze-thaw cycles for decellularization of tendon tissue - a pilot study
Abstract
Background: Decellularization of tendon tissue plays a pivotal role in current tissue engineering approaches for in vitro research as well as for translation of graft-based tendon restoration into clinics. Automation of essential decellularization steps like freeze-thawing is crucial for the development of more standardized decellularization protocols and commercial graft production under good manufacturing practice (GMP) conditions in the future.
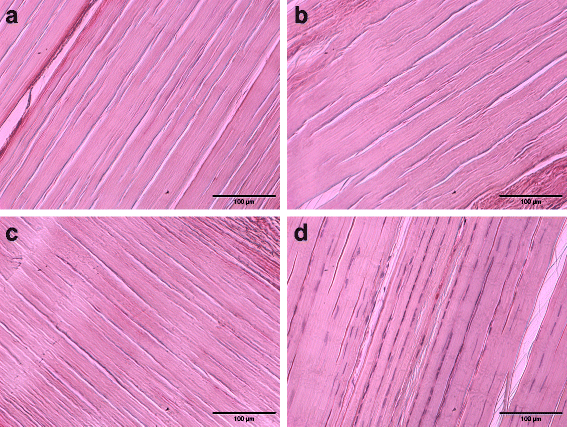
Methods: In this study, a liquid nitrogen-based controlled rate freezer was utilized for automation of repeated freeze-thawing for decellularization of equine superficial digital flexor tendons. Additional tendon specimens underwent manually performed freeze-thaw cycles based on an established procedure. Tendon decellularization was completed by using non-ionic detergent treatment (Triton X-100). Effectiveness of decellularization was assessed by residual nuclei count and calculation of DNA content. Cytocompatibility was evaluated by culturing allogeneic adipose tissue-derived mesenchymal stromal cells on the tendon scaffolds.
Results: There were no significant differences in decellularization effectiveness between samples decellularized by the automated freeze-thaw procedure and samples that underwent manual freeze-thaw cycles. Further, we inferred no significant differences in the effectiveness of decellularization between two different cooling and heating rates applied in the automated freeze-thaw process. Both the automated protocols and the manually performed protocol resulted in roughly 2% residual nuclei and 13% residual DNA content. Successful cell culture was achieved with samples decellularized by automated freeze-thawing as well as with tendon samples decellularized by manually performed freeze-thaw cycles.
Conclusions: Automated freeze-thaw cycles performed by using a liquid nitrogen-based controlled rate freezer were as effective as previously described manual freeze-thaw procedures for decellularization of equine superficial digital flexor tendons. The automation of this key procedure in decellularization of large tendon samples is an important step towards the processing of large sample quantities under standardized conditions. Furthermore, with a view to the production of commercially available tendon graft-based materials for application in human and veterinary medicine, the automation of key procedural steps is highly required to develop manufacturing processes under GMP conditions.
Keywords: Automation; Controlled rate freezer; Decellularization; Horse; Regenerative medicine; Tendon; Tissue engineering.
Figures




Similar articles
-
Decellularization of Large Tendon Specimens: Combination of Manually Performed Freeze-Thaw Cycles and Detergent Treatment.Methods Mol Biol. 2018;1577:227-237. doi: 10.1007/7651_2017_49. Methods Mol Biol. 2018. PMID: 28702884
-
Freeze-thaw cycles enhance decellularization of large tendons.Tissue Eng Part C Methods. 2014 Apr;20(4):276-84. doi: 10.1089/ten.TEC.2012.0760. Epub 2013 Sep 21. Tissue Eng Part C Methods. 2014. PMID: 23879725 Free PMC article.
-
Combination of freeze-thaw with detergents: A promising approach to the decellularization of porcine carotid arteries.Biomed Mater Eng. 2019;30(2):191-205. doi: 10.3233/BME-191044. Biomed Mater Eng. 2019. PMID: 30741667
-
The trend of allogeneic tendon decellularization: literature review.Cell Tissue Bank. 2024 Mar;25(1):357-367. doi: 10.1007/s10561-023-10097-x. Epub 2023 Jun 24. Cell Tissue Bank. 2024. PMID: 37355504 Review.
-
[Research progress of decellularization and application in tissue engineering].Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2013 Aug;27(8):950-4. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2013. PMID: 24171349 Review. Chinese.
Cited by
-
A 3D Dynamic In Vitro Model of Inflammatory Tendon Disease.Methods Mol Biol. 2021;2269:167-174. doi: 10.1007/978-1-0716-1225-5_12. Methods Mol Biol. 2021. PMID: 33687679
-
Engineering of Tracheal Grafts Based on Recellularization of Laser-Engraved Human Airway Cartilage Substrates.Cartilage. 2022 Jan-Mar;13(1):19476035221075951. doi: 10.1177/19476035221075951. Cartilage. 2022. PMID: 35189712 Free PMC article.
-
Decellularization of Dense Regular Connective Tissue-Cellular and Molecular Modification with Applications in Regenerative Medicine.Cells. 2023 Sep 16;12(18):2293. doi: 10.3390/cells12182293. Cells. 2023. PMID: 37759515 Free PMC article. Review.
-
Different Decellularization Methods in Bovine Lung Tissue Reveals Distinct Biochemical Composition, Stiffness, and Viscoelasticity in Reconstituted Hydrogels.ACS Appl Bio Mater. 2023 Feb 20;6(2):793-805. doi: 10.1021/acsabm.2c00968. Epub 2023 Feb 2. ACS Appl Bio Mater. 2023. PMID: 36728815 Free PMC article.
-
Rho/ROCK Inhibition Promotes TGF-β3-Induced Tenogenic Differentiation in Mesenchymal Stromal Cells.Stem Cells Int. 2021 Oct 8;2021:8284690. doi: 10.1155/2021/8284690. eCollection 2021. Stem Cells Int. 2021. PMID: 34659420 Free PMC article.
References
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

