Potential Deaths Averted and Serious Adverse Events Incurred From Adoption of the SPRINT (Systolic Blood Pressure Intervention Trial) Intensive Blood Pressure Regimen in the United States: Projections From NHANES (National Health and Nutrition Examination Survey)
- PMID: 28193605
- PMCID: PMC5404971
- DOI: 10.1161/CIRCULATIONAHA.116.025322
Potential Deaths Averted and Serious Adverse Events Incurred From Adoption of the SPRINT (Systolic Blood Pressure Intervention Trial) Intensive Blood Pressure Regimen in the United States: Projections From NHANES (National Health and Nutrition Examination Survey)
Abstract
Background: SPRINT (Systolic Blood Pressure Intervention Trial) demonstrated a 27% reduction in all-cause mortality with a systolic blood pressure (SBP) goal of <120 versus <140 mm Hg among US adults at high cardiovascular disease risk but without diabetes mellitus, stroke, or heart failure. To quantify the potential benefits and risks of SPRINT intensive goal implementation, we estimated the deaths prevented and excess serious adverse events incurred if the SPRINT intensive SBP treatment goal were implemented in all eligible US adults.
Methods: SPRINT eligibility criteria were applied to the 1999 to 2006 National Health and Nutrition Examination Survey and linked with the National Death Index through December 2011. SPRINT eligibility included age ≥50 years, SBP of 130 to 180 mm Hg (depending on the number of antihypertensive medications being taken), and high cardiovascular disease risk. Exclusion criteria were diabetes mellitus, history of stroke, >1 g proteinuria, heart failure, estimated glomerular filtration rate <20 mL·min-1·1.73 m-2, or dialysis. Annual mortality rates were calculated by dividing the Kaplan-Meier 5-year mortality by 5. Hazard ratios for all-cause mortality and heart failure and absolute risks for serious adverse events in SPRINT were used to estimate the number of potential deaths and heart failure cases prevented and serious adverse events incurred with intensive SBP treatment.
Results: The mean age was 68.6 years, and 83.2% and 7.4% were non-Hispanic white and non-Hispanic black, respectively. The annual mortality rate was 2.20% (95% confidence interval [CI], 1.91-2.48), and intensive SBP treatment was projected to prevent ≈107 500 deaths per year (95% CI, 93 300-121 200) and give rise to 56 100 (95% CI, 50 800-61 400) episodes of hypotension, 34 400 (95% CI, 31 200-37 600) episodes of syncope, 43 400 (95% CI, 39 400-47 500) serious electrolyte disorders, and 88 700 (95% CI, 80 400-97 000) cases of acute kidney injury per year. The analysis-of-extremes approach indicated that the range of estimated lower- and upper-bound number of deaths prevented per year with intensive SBP control was 34 600 to 179 600. Intensive SBP control was projected to prevent 46 100 (95% CI, 41 800-50 400) cases of heart failure annually.
Conclusions: If fully implemented in eligible US adults, intensive SBP treatment could prevent ≈107 500 deaths per year. A consequence of this treatment strategy, however, could be an increase in serious adverse events.
Keywords: blood pressure; hypertension; treatment outcome.
© 2017 American Heart Association, Inc.
Conflict of interest statement
APB: Receives research support from Novartis not related to the current project.
HK: No conflicts of interest to disclose
RH: No conflicts of interest to disclose
RK: No conflicts of interest to disclose
SB: No conflicts of interest to disclose
AKC: No conflicts of interest to disclose
VKB: No conflicts of interest to disclose
GC: No conflicts of interest to disclose
JY: No conflicts of interest to disclose
AEM: No conflicts of interest to disclose
RD: No conflicts of interest to disclose
PM: No conflicts of interest to disclose
RC: No conflicts of interest to disclose
Figures

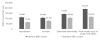
Comment in
-
Hypertension Treatment and Outcomes in the Era of Population Health, Coordinated Care, and Medicare Access and CHIP Reauthorization Act (MACRA).Circulation. 2017 Apr 25;135(17):1629-1631. doi: 10.1161/CIRCULATIONAHA.117.027604. Circulation. 2017. PMID: 28438804 No abstract available.
-
Letter by Donzelli Regarding Article, "Potential Deaths Averted and Serious Adverse Events Incurred From Adoption of the SPRINT (Systolic Blood Pressure Intervention Trial) Intensive Blood Pressure Regimen in the United States: Projections From NHANES (National Health and Nutrition Examination Survey)".Circulation. 2017 Sep 19;136(12):1170-1171. doi: 10.1161/CIRCULATIONAHA.117.029124. Circulation. 2017. PMID: 28923907 No abstract available.
-
Letter by Koh Regarding Article, "Potential Deaths Averted and Serious Adverse Events Incurred From Adoption of the SPRINT (Systolic Blood Pressure Intervention Trial) Intensive Blood Pressure Regimen in the United States: Projections From NHANES (National Health and Nutrition Examination Survey)".Circulation. 2017 Sep 19;136(12):1172-1173. doi: 10.1161/CIRCULATIONAHA.117.029629. Circulation. 2017. PMID: 28923908 No abstract available.
-
Response by Bress et al to Letters Regarding Article, "Potential Deaths Averted and Serious Adverse Events Incurred From Adoption of the SPRINT (Systolic Blood Pressure Intervention Trial) Intensive Blood Pressure Regimen in the United States: Projections from NHANES (National Health and Nutrition Examination Survey)".Circulation. 2017 Sep 19;136(12):1174-1175. doi: 10.1161/CIRCULATIONAHA.117.029937. Circulation. 2017. PMID: 28923909 Free PMC article. No abstract available.
References
-
- Lim SS, Vos T, Flaxman AD, Danaei G, Shibuya K, Adair-Rohani H, Amann M, Anderson HR, Andrews KG, Aryee M, Atkinson C, Bacchus LJ, Bahalim AN, Balakrishnan K, Balmes J, Barker-Collo S, Baxter A, Bell ML, Blore JD, Blyth F, Bonner C, Borges G, Bourne R, Boussinesq M, Brauer M, Brooks P, Bruce NG, Brunekreef B, Bryan-Hancock C, Bucello C, Buchbinder R, Bull F, Burnett RT, Byers TE, Calabria B, Carapetis J, Carnahan E, Chafe Z, Charlson F, Chen H, Chen JS, Cheng AT, Child JC, Cohen A, Colson KE, Cowie BC, Darby S, Darling S, Davis A, Degenhardt L, Dentener F, Des Jarlais DC, Devries K, Dherani M, Ding EL, Dorsey ER, Driscoll T, Edmond K, Ali SE, Engell RE, Erwin PJ, Fahimi S, Falder G, Farzadfar F, Ferrari A, Finucane MM, Flaxman S, Fowkes FG, Freedman G, Freeman MK, Gakidou E, Ghosh S, Giovannucci E, Gmel G, Graham K, Grainger R, Grant B, Gunnell D, Gutierrez HR, Hall W, Hoek HW, Hogan A, Hosgood HD, 3rd, Hoy D, Hu H, Hubbell BJ, Hutchings SJ, Ibeanusi SE, Jacklyn GL, Jasrasaria R, Jonas JB, Kan H, Kanis JA, Kassebaum N, Kawakami N, Khang YH, Khatibzadeh S, Khoo JP, Kok C, Laden F, Lalloo R, Lan Q, Lathlean T, Leasher JL, Leigh J, Li Y, Lin JK, Lipshultz SE, London S, Lozano R, Lu Y, Mak J, Malekzadeh R, Mallinger L, Marcenes W, March L, Marks R, Martin R, McGale P, McGrath J, Mehta S, Mensah GA, Merriman TR, Micha R, Michaud C, Mishra V, Mohd Hanafiah K, Mokdad AA, Morawska L, Mozaffarian D, Murphy T, Naghavi M, Neal B, Nelson PK, Nolla JM, Norman R, Olives C, Omer SB, Orchard J, Osborne R, Ostro B, Page A, Pandey KD, Parry CD, Passmore E, Patra J, Pearce N, Pelizzari PM, Petzold M, Phillips MR, Pope D, Pope CA, 3rd, Powles J, Rao M, Razavi H, Rehfuess EA, Rehm JT, Ritz B, Rivara FP, Roberts T, Robinson C, Rodriguez-Portales JA, Romieu I, Room R, Rosenfeld LC, Roy A, Rushton L, Salomon JA, Sampson U, Sanchez-Riera L, Sanman E, Sapkota A, Seedat S, Shi P, Shield K, Shivakoti R, Singh GM, Sleet DA, Smith E, Smith KR, Stapelberg NJ, Steenland K, Stockl H, Stovner LJ, Straif K, Straney L, Thurston GD, Tran JH, Van Dingenen R, van Donkelaar A, Veerman JL, Vijayakumar L, Weintraub R, Weissman MM, White RA, Whiteford H, Wiersma ST, Wilkinson JD, Williams HC, Williams W, Wilson N, Woolf AD, Yip P, Zielinski JM, Lopez AD, Murray CJ, Ezzati M, AlMazroa MA, Memish ZA. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2224–60. - PMC - PubMed
-
- Lewington S, Clarke R, Qizilbash N, Peto R, Collins R Prospective Studies C. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360:1903–13. - PubMed
-
- James PA, Oparil S, Carter BL, Cushman WC, Dennison-Himmelfarb C, Handler J, Lackland DT, LeFevre ML, MacKenzie TD, Ogedegbe O, Smith SC, Jr, Svetkey LP, Taler SJ, Townsend RR, Wright JT, Jr, Narva AS, Ortiz E. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8) JAMA. 2014;311:507–20. - PubMed
-
- Cushman WC, Evans GW, Byington RP, Goff DC, Jr, Grimm RH, Jr, Cutler JA, Simons-Morton DG, Basile JN, Corson MA, Probstfield JL, Katz L, Peterson KA, Friedewald WT, Buse JB, Bigger JT, Gerstein HC, Ismail-Beigi F. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med. 2010;362:1575–85. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

