Safe and Effective Sarcoma Therapy through Bispecific Targeting of EGFR and uPAR
- PMID: 28193671
- PMCID: PMC5418099
- DOI: 10.1158/1535-7163.MCT-16-0637
Safe and Effective Sarcoma Therapy through Bispecific Targeting of EGFR and uPAR
Abstract
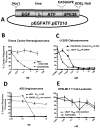
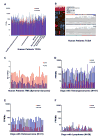
Sarcomas differ from carcinomas in their mesenchymal origin. Therapeutic advancements have come slowly, so alternative drugs and models are urgently needed. These studies report a new drug for sarcomas that simultaneously targets both tumor and tumor neovasculature. eBAT is a bispecific angiotoxin consisting of truncated, deimmunized Pseudomonas exotoxin fused to EGF and the amino terminal fragment of urokinase. Here, we study the drug in an in vivo "ontarget" companion dog trial as eBAT effectively kills canine hemangiosarcoma and human sarcoma cells in vitro We reasoned the model has value due to the common occurrence of spontaneous sarcomas in dogs and a limited lifespan allowing for rapid accrual and data collection. Splenectomized dogs with minimal residual disease were given one cycle of eBAT followed by adjuvant doxorubicin in an adaptive dose-finding, phase I-II study of 23 dogs with spontaneous, stage I-II, splenic hemangiosarcoma. eBAT improved 6-month survival from <40% in a comparison population to approximately 70% in dogs treated at a biologically active dose (50 μg/kg). Six dogs were long-term survivors, living >450 days. eBAT abated expected toxicity associated with EGFR targeting, a finding supported by mouse studies. Urokinase plasminogen activator receptor and EGFR are targets for human sarcomas, so thorough evaluation is crucial for validation of the dog model. Thus, we validated these markers for human sarcoma targeting in the study of 212 human and 97 canine sarcoma samples. Our results support further translation of eBAT for human patients with sarcomas and perhaps other EGFR-expressing malignancies. Mol Cancer Ther; 16(5); 956-65. ©2017 AACR.
©2017 American Association for Cancer Research.
Conflict of interest statement
Figures


References
-
- Cassier PA, Polivka V, Judson I, Soria JC, Penel N, Marsoni S, et al. Outcome of patients with sarcoma and other mesenchymal tumours participating in phase I trials: a subset analysis of a European Phase I database. Ann Oncol. 2014;25:1222–8. - PubMed
-
- Linch M, Miah AB, Thway K, Judson IR, Benson C. Systemic treatment of soft-tissue sarcoma-gold standard and novel therapies. Nat Rev Clin Oncol. 2014;11:187–202. - PubMed
-
- Ng VY, Scharschmidt TJ, Mayerson JL, Fisher JL. Incidence and survival in sarcoma in the United States: a focus on musculoskeletal lesions. Anticancer Res. 2013;33:2597–2604. - PubMed
-
- Borden EC, Baker LH, Bell RS, Bramwell V, Demetri VGD, Eisenberg BL, et al. Soft tissue sarcomas of adults: state of the translational science. Clin Cancer Res. 2003;9:1941–1956. - PubMed
-
- Frith AE, Hirbe AC, Van Tine BA. Novel pathways and molecular targets for the treatment of sarcoma. Curr Oncol Rep. 2013;15(4):378–85. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

