Paraventricular Thalamus Balances Danger and Reward
- PMID: 28193686
- PMCID: PMC6596734
- DOI: 10.1523/JNEUROSCI.3320-16.2017
Paraventricular Thalamus Balances Danger and Reward
Abstract
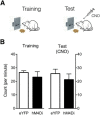
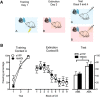
Foraging animals balance the need to seek food and energy against the accompanying dangers of injury and predation. To do so, they rely on learning systems encoding reward and danger. Whereas much is known about these separate learning systems, little is known about how they interact to shape and guide behavior. Here we show a key role for the rat paraventricular nucleus of the thalamus (PVT), a nucleus of the dorsal midline thalamus, in this interaction. First, we show behavioral competition between reward and danger: the opportunity to seek food reward negatively modulates expression of species-typical defensive behavior. Then, using a chemogenetic approach expressing the inhibitory hM4Di designer receptor exclusively activated by a designer drug in PVT neurons, we show that the PVT is central to this behavioral competition. Chemogenetic PVT silencing biases behavior toward either defense or reward depending on the experimental conditions, but does not consistently favor expression of one over the other. This bias could not be attributed to changes in fear memory retrieval, learned safety, or memory interference. Rather, our results demonstrate that the PVT is essential for balancing conflicting behavioral tendencies toward danger and reward, enabling adaptive responding under this basic selection pressure.SIGNIFICANCE STATEMENT Among the most basic survival problems faced by animals is balancing the need to seek food and energy against the accompanying dangers of injury and predation. Although much is known about the brain mechanisms that underpin learning about reward and danger, little is known about how these interact to solve basic survival problems. Here we show competition between defensive (to avoid predatory detection) and approach (to obtain food) behavior. We show that the paraventricular thalamus, a nucleus of the dorsal midline thalamus, is integral to this behavioral competition. The paraventricular thalamus balances the competing behavioral demands of danger and reward, enabling adaptive responding under this selection pressure.
Keywords: competition; danger; paraventricular thalamus; reward.
Copyright © 2017 the authors 0270-6474/17/373018-12$15.00/0.
Figures





References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
