Regulation of Hematopoiesis and Hematological Disease by TGF-β Family Signaling Molecules
- PMID: 28193723
- PMCID: PMC5585852
- DOI: 10.1101/cshperspect.a027987
Regulation of Hematopoiesis and Hematological Disease by TGF-β Family Signaling Molecules
Abstract
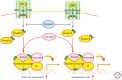
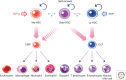
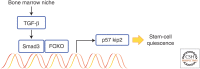
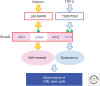
Throughout the lifetime of an individual, hematopoietic stem cells (HSCs) maintain the homeostasis of normal hematopoiesis through the precise generation of mature blood cells. Numerous genetic studies in mice have shown that stem-cell quiescence is critical for sustaining primitive long-term HSCs in vivo. In this review, we first examine the crucial roles of transforming growth factor β (TGF-β) and related signaling molecules in not only regulating the well-known cytostatic effects of these molecules but also governing the self-renewal capacity of HSCs in their in vivo microenvironmental niche. Second, we discuss the current evidence indicating that TGF-β signaling has a dual function in disorders of the hematopoietic system. In particular, we examine the paradox that, although intrinsic TGF-β signaling is essential for regulating the survival and resistance to therapy of chronic myelogenous leukemia (CML) stem cells, genetic changes that abrogate TGF-β signaling can lead to the development of several hematological malignancies.
Copyright © 2017 Cold Spring Harbor Laboratory Press; all rights reserved.
Figures

References
-
- Araki M, Yang Y, Masubuchi N, Hironaka Y, Takei H, Morishita S, Mizukami Y, Kan S, Shirane S, Edahiro Y, et al. 2016. Activation of the thrombopoietin receptor by mutant calreticulin in CALR-mutant myeloproliferative neoplasms. Blood 127: 1307–1316. - PubMed
-
- Arber DA, Orazi A, Hasserjian R, Thiele J, Borowitz MJ, Le Beau MM, Bloomfield CD, Cazzola M, Vardiman JW. 2016. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood 127: 2391–2405. - PubMed
-
- Arnulf B, Villemain A, Nicot C, Mordelet E, Charneau P, Kersual J, Zermati Y, Mauviel A, Bazarbachi A, Hermine O. 2002. Human T-cell lymphotropic virus oncoprotein Tax represses TGF-β1 signaling in human T cells via c-Jun activation: A potential mechanism of HTLV-I leukemogenesis. Blood 100: 4129–4138. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
