Monocyte Subsets Coregulate Inflammatory Responses by Integrated Signaling through TNF and IL-6 at the Endothelial Cell Interface
- PMID: 28193827
- PMCID: PMC5357784
- DOI: 10.4049/jimmunol.1601281
Monocyte Subsets Coregulate Inflammatory Responses by Integrated Signaling through TNF and IL-6 at the Endothelial Cell Interface
Abstract
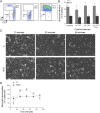
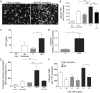
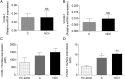
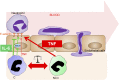
Two major monocyte subsets, CD14+CD16- (classical) and CD14+/dimCD16+ (nonclassical/intermediate), have been described. Each has different functions ascribed in its interactions with vascular endothelial cells (EC), including migration and promoting inflammation. Although monocyte subpopulations have been studied in isolated systems, their influence on EC and on the course of inflammation has been ignored. In this study, using unstimulated or cytokine-activated EC, we observed significant differences in the recruitment, migration, and reverse migration of human monocyte subsets. Associated with this, and based on their patterns of cytokine secretion, there was a difference in their capacity to activate EC and support the secondary recruitment of flowing neutrophils. High levels of TNF were detected in cocultures with nonclassical/intermediate monocytes, the blockade of which significantly reduced neutrophil recruitment. In contrast, classical monocytes secreted high levels of IL-6, the blockade of which resulted in increased neutrophil recruitment. When cocultures contained both monocyte subsets, or when conditioned supernatant from classical monocytes cocultures (IL-6hi) was added to nonclassical/intermediate monocyte cocultures (TNFhi), the activating effects of TNF were dramatically reduced, implying that when present, the anti-inflammatory activities of IL-6 were dominant over the proinflammatory activities of TNF. These changes in neutrophil recruitment could be explained by regulation of E-selectin on the cocultured EC. This study suggests that recruited human monocyte subsets trigger a regulatory pathway of cytokine-mediated signaling at the EC interface, and we propose that this is a mechanism for limiting the phlogistic activity of newly recruited monocytes.
Copyright © 2017 The Authors.
Figures

References
-
- Bobryshev Y. V. 2006. Monocyte recruitment and foam cell formation in atherosclerosis. Micron 37: 208–222. - PubMed
-
- Ross R. 1995. Cell biology of atherosclerosis. Annu. Rev. Physiol. 57: 791–804. - PubMed
-
- Baeten D., Boots A. M., Steenbakkers P. G., Elewaut D., Bos E., Verheijden G. F., Berheijden G., Miltenburg A. M., Rijnders A. W., Veys E. M., De Keyser F. 2000. Human cartilage gp-39+,CD16+ monocytes in peripheral blood and synovium: correlation with joint destruction in rheumatoid arthritis. Arthritis Rheum. 43: 1233–1243. - PubMed
-
- Kawanaka N., Yamamura M., Aita T., Morita Y., Okamoto A., Kawashima M., Iwahashi M., Ueno A., Ohmoto Y., Makino H. 2002. CD14+,CD16+ blood monocytes and joint inflammation in rheumatoid arthritis. Arthritis Rheum. 46: 2578–2586. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

