Vulnerability of primitive human placental trophoblast to Zika virus
- PMID: 28193876
- PMCID: PMC5338554
- DOI: 10.1073/pnas.1616097114
Vulnerability of primitive human placental trophoblast to Zika virus
Abstract
Infection of pregnant women by Asian lineage strains of Zika virus (ZIKV) has been linked to brain abnormalities in their infants, yet it is uncertain when during pregnancy the human conceptus is most vulnerable to the virus. We have examined two models to study susceptibility of human placental trophoblast to ZIKV: cytotrophoblast and syncytiotrophoblast derived from placental villi at term and colonies of trophoblast differentiated from embryonic stem cells (ESC). The latter appear to be analogous to the primitive placenta formed during implantation. The cells from term placentas, which resist infection, do not express genes encoding most attachment factors implicated in ZIKV entry but do express many genes associated with antiviral defense. By contrast, the ESC-derived trophoblasts possess a wide range of attachment factors for ZIKV entry and lack components of a robust antiviral response system. These cells, particularly areas of syncytiotrophoblast within the colonies, quickly become infected, produce infectious virus and undergo lysis within 48 h after exposure to low titers (multiplicity of infection > 0.07) of an African lineage strain (MR766 Uganda: ZIKVU) considered to be benign with regards to effects on fetal development. Unexpectedly, lytic effects required significantly higher titers of the presumed more virulent FSS13025 Cambodia (ZIKVC). Our data suggest that the developing fetus might be most vulnerable to ZIKV early in the first trimester before a protective zone of mature villous trophoblast has been established. Additionally, MR766 is highly trophic toward primitive trophoblast, which may put the early conceptus of an infected mother at high risk for destruction.
Keywords: Zika virus; embryonic stem cell; placenta; pregnancy; trophoblast.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


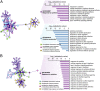
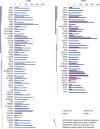
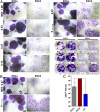
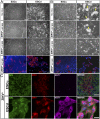
References
-
- Delorme-Axford E, Sadovsky Y, Coyne CB. The placenta as a barrier to viral infections. Ann Rev Virol. 2014;1(1):133–146. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

