TMEM175 deficiency impairs lysosomal and mitochondrial function and increases α-synuclein aggregation
- PMID: 28193887
- PMCID: PMC5338534
- DOI: 10.1073/pnas.1616332114
TMEM175 deficiency impairs lysosomal and mitochondrial function and increases α-synuclein aggregation
Abstract
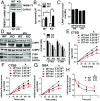
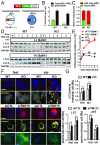
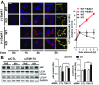
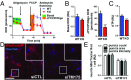
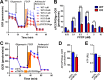
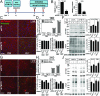
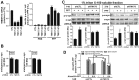
Parkinson disease (PD) is a neurodegenerative disorder pathologically characterized by nigrostriatal dopamine neuron loss and the postmortem presence of Lewy bodies, depositions of insoluble α-synuclein, and other proteins that likely contribute to cellular toxicity and death during the disease. Genetic and biochemical studies have implicated impaired lysosomal and mitochondrial function in the pathogenesis of PD. Transmembrane protein 175 (TMEM175), the lysosomal K+ channel, is centered under a major genome-wide association studies peak for PD, making it a potential candidate risk factor for the disease. To address the possibility that variation in TMEM175 could play a role in PD pathogenesis, TMEM175 function was investigated in a neuronal model system. Studies confirmed that TMEM175 deficiency results in unstable lysosomal pH, which led to decreased lysosomal catalytic activity, decreased glucocerebrosidase activity, impaired autophagosome clearance by the lysosome, and decreased mitochondrial respiration. Moreover, TMEM175 deficiency in rat primary neurons resulted in increased susceptibility to exogenous α-synuclein fibrils. Following α-synuclein fibril treatment, neurons deficient in TMEM175 were found to have increased phosphorylated and detergent-insoluble α-synuclein deposits. Taken together, data from these studies suggest that TMEM175 plays a direct and critical role in lysosomal and mitochondrial function and PD pathogenesis and highlight this ion channel as a potential therapeutic target for treating PD.
Keywords: Parkinson disease; TMEM175; lysosome; mitochondria; α-synuclein.
Conflict of interest statement
Conflict of interest statement: All of the authors are employed by Merck & Co.
Figures

References
-
- Zhu Z, et al. Integration of summary data from GWAS and eQTL studies predicts complex trait gene targets. Nat Genet. 2016;48(5):481–487. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

