Multiple-scale neuroendocrine signals connect brain and pituitary hormone rhythms
- PMID: 28193889
- PMCID: PMC5338546
- DOI: 10.1073/pnas.1616864114
Multiple-scale neuroendocrine signals connect brain and pituitary hormone rhythms
Abstract
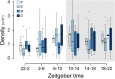
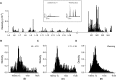
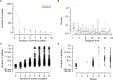
Small assemblies of hypothalamic "parvocellular" neurons release their neuroendocrine signals at the median eminence (ME) to control long-lasting pituitary hormone rhythms essential for homeostasis. How such rapid hypothalamic neurotransmission leads to slowly evolving hormonal signals remains unknown. Here, we show that the temporal organization of dopamine (DA) release events in freely behaving animals relies on a set of characteristic features that are adapted to the dynamic dopaminergic control of pituitary prolactin secretion, a key reproductive hormone. First, locally generated DA release signals are organized over more than four orders of magnitude (0.001 Hz-10 Hz). Second, these DA events are finely tuned within and between frequency domains as building blocks that recur over days to weeks. Third, an integration time window is detected across the ME and consists of high-frequency DA discharges that are coordinated within the minutes range. Thus, a hierarchical combination of time-scaled neuroendocrine signals displays local-global integration to connect brain-pituitary rhythms and pace hormone secretion.
Keywords: dopamine; hypothalamus; neuronal networks; prolactin; rhythms.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
Ovarian steroids influence the activity of neuroendocrine dopaminergic neurons.Brain Res. 2000 Oct 6;879(1-2):139-47. doi: 10.1016/s0006-8993(00)02763-3. Brain Res. 2000. PMID: 11011015
-
Prolactin activates all three populations of hypothalamic neuroendocrine dopaminergic neurons in ovariectomized rats.Brain Res. 1999 Aug 7;837(1-2):236-41. doi: 10.1016/s0006-8993(99)01667-4. Brain Res. 1999. PMID: 10434008
-
Immunoneutralization of prolactin prevents stimulatory feedback of prolactin on hypothalamic neuroendocrine dopaminergic neurons.Endocrine. 2000 Jun;12(3):333-7. doi: 10.1385/ENDO:12:3:333. Endocrine. 2000. PMID: 10963056
-
Physiological significance of the rhythmic secretion of hypothalamic and pituitary hormones.Prog Brain Res. 2010;181:111-26. doi: 10.1016/S0079-6123(08)81007-2. Prog Brain Res. 2010. PMID: 20478435 Review.
-
Circadian and ultradian patterns of HPA-axis activity in rodents: Significance for brain functionality.Best Pract Res Clin Endocrinol Metab. 2017 Oct;31(5):445-457. doi: 10.1016/j.beem.2017.09.001. Epub 2017 Sep 20. Best Pract Res Clin Endocrinol Metab. 2017. PMID: 29223280 Review.
Cited by
-
Protocol for in vivo recording of neural activity in deep structures of mice brain via gradient lenses by calcium imaging.STAR Protoc. 2025 Mar 21;6(1):103534. doi: 10.1016/j.xpro.2024.103534. Epub 2024 Dec 20. STAR Protoc. 2025. PMID: 39708323 Free PMC article.
-
Dopamine Release Dynamics in the Tuberoinfundibular Dopamine System.J Neurosci. 2019 May 22;39(21):4009-4022. doi: 10.1523/JNEUROSCI.2339-18.2019. Epub 2019 Feb 19. J Neurosci. 2019. PMID: 30782976 Free PMC article.
-
Trpc5 deficiency causes hypoprolactinemia and altered function of oscillatory dopamine neurons in the arcuate nucleus.Proc Natl Acad Sci U S A. 2019 Jul 23;116(30):15236-15243. doi: 10.1073/pnas.1905705116. Epub 2019 Jul 8. Proc Natl Acad Sci U S A. 2019. PMID: 31285329 Free PMC article.
-
Neural substrates underlying rhythmic coupling of female reproductive and thermoregulatory circuits.Front Physiol. 2023 Sep 11;14:1254287. doi: 10.3389/fphys.2023.1254287. eCollection 2023. Front Physiol. 2023. PMID: 37753455 Free PMC article. Review.
-
Multi-dimensional oscillatory activity of mouse GnRH neurons in vivo.Elife. 2025 Jan 8;13:RP100856. doi: 10.7554/eLife.100856. Elife. 2025. PMID: 39773874 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

