Kv1.1 channelopathy abolishes presynaptic spike width modulation by subthreshold somatic depolarization
- PMID: 28193892
- PMCID: PMC5338558
- DOI: 10.1073/pnas.1608763114
Kv1.1 channelopathy abolishes presynaptic spike width modulation by subthreshold somatic depolarization
Abstract
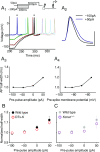
Although action potentials propagate along axons in an all-or-none manner, subthreshold membrane potential fluctuations at the soma affect neurotransmitter release from synaptic boutons. An important mechanism underlying analog-digital modulation is depolarization-mediated inactivation of presynaptic Kv1-family potassium channels, leading to action potential broadening and increased calcium influx. Previous studies have relied heavily on recordings from blebs formed after axon transection, which may exaggerate the passive propagation of somatic depolarization. We recorded instead from small boutons supplied by intact axons identified with scanning ion conductance microscopy in primary hippocampal cultures and asked how distinct potassium channels interact in determining the basal spike width and its modulation by subthreshold somatic depolarization. Pharmacological or genetic deletion of Kv1.1 broadened presynaptic spikes without preventing further prolongation by brief depolarizing somatic prepulses. A heterozygous mouse model of episodic ataxia type 1 harboring a dominant Kv1.1 mutation had a similar broadening effect on basal spike shape as deletion of Kv1.1; however, spike modulation by somatic prepulses was abolished. These results argue that the Kv1.1 subunit is not necessary for subthreshold modulation of spike width. However, a disease-associated mutant subunit prevents the interplay of analog and digital transmission, possibly by disrupting the normal stoichiometry of presynaptic potassium channels.
Keywords: channelopathy; potassium channel; synaptic transmission.
Conflict of interest statement
The authors declare no conflict of interest.
Figures













References
-
- Alle H, Geiger JRP. Combined analog and action potential coding in hippocampal mossy fibers. Science. 2006;311(5765):1290–1293. - PubMed
-
- Shu Y, Hasenstaub A, Duque A, Yu Y, McCormick DA. Modulation of intracortical synaptic potentials by presynaptic somatic membrane potential. Nature. 2006;441(7094):761–765. - PubMed
-
- Debanne D, Bialowas A, Rama S. What are the mechanisms for analogue and digital signalling in the brain? Nat Rev Neurosci. 2013;14(1):63–69. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
- BB/D018595/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- MR/L01095X/1/MRC_/Medical Research Council/United Kingdom
- G0801316/MRC_/Medical Research Council/United Kingdom
- G0200373/MRC_/Medical Research Council/United Kingdom
- G0802158/MRC_/Medical Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

