Regulatory cascade and biological activity of Beauveria bassiana oosporein that limits bacterial growth after host death
- PMID: 28193896
- PMCID: PMC5338512
- DOI: 10.1073/pnas.1616543114
Regulatory cascade and biological activity of Beauveria bassiana oosporein that limits bacterial growth after host death
Abstract
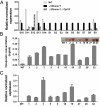
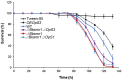
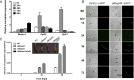
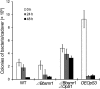
The regulatory network and biological functions of the fungal secondary metabolite oosporein have remained obscure. Beauveria bassiana has evolved the ability to parasitize insects and outcompete microbial challengers for assimilation of host nutrients. A novel zinc finger transcription factor, BbSmr1 (B. bassiana secondary metabolite regulator 1), was identified in a screen for oosporein overproduction. Deletion of Bbsmr1 resulted in up-regulation of the oosporein biosynthetic gene cluster (OpS genes) and constitutive oosporein production. Oosporein production was abolished in double mutants of Bbsmr1 and a second transcription factor, OpS3, within the oosporein gene cluster (ΔBbsmr1ΔOpS3), indicating that BbSmr1 acts as a negative regulator of OpS3 expression. Real-time quantitative PCR and a GFP promoter fusion construct of OpS1, the oosporein polyketide synthase, indicated that OpS1 is expressed mainly in insect cadavers at 24-48 h after death. Bacterial colony analysis in B. bassiana-infected insect hosts revealed increasing counts until host death, with a dramatic decrease (∼90%) after death that correlated with oosporein production. In vitro studies verified the inhibitory activity of oosporein against bacteria derived from insect cadavers. These results suggest that oosporein acts as an antimicrobial compound to limit microbial competition on B. bassiana-killed hosts, allowing the fungus to maximally use host nutrients to grow and sporulate on infected cadavers.
Keywords: Beauveria bassiana; biological role; fungal–bacterial competition; oosporein; transcription factor.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Transcription Factors BbPacC and Bbmsn2 Jointly Regulate Oosporein Production in Beauveria bassiana.Microbiol Spectr. 2022 Dec 21;10(6):e0311822. doi: 10.1128/spectrum.03118-22. Epub 2022 Nov 23. Microbiol Spectr. 2022. PMID: 36416546 Free PMC article.
-
Fungal biosynthesis of the bibenzoquinone oosporein to evade insect immunity.Proc Natl Acad Sci U S A. 2015 Sep 8;112(36):11365-70. doi: 10.1073/pnas.1503200112. Epub 2015 Aug 24. Proc Natl Acad Sci U S A. 2015. PMID: 26305932 Free PMC article.
-
Hydrophobins contribute to root colonization and stress responses in the rhizosphere-competent insect pathogenic fungus Beauveria bassiana.Microbiology (Reading). 2018 Apr;164(4):517-528. doi: 10.1099/mic.0.000644. Epub 2018 Mar 8. Microbiology (Reading). 2018. PMID: 29517481
-
Molecular Genetics of Beauveria bassiana Infection of Insects.Adv Genet. 2016;94:165-249. doi: 10.1016/bs.adgen.2015.11.003. Epub 2016 Feb 11. Adv Genet. 2016. PMID: 27131326 Review.
-
The Toxins of Beauveria bassiana and the Strategies to Improve Their Virulence to Insects.Front Microbiol. 2021 Aug 26;12:705343. doi: 10.3389/fmicb.2021.705343. eCollection 2021. Front Microbiol. 2021. PMID: 34512581 Free PMC article. Review.
Cited by
-
Production of Helvolic Acid in Metarhizium Contributes to Fungal Infection of Insects by Bacteriostatic Inhibition of the Host Cuticular Microbiomes.Microbiol Spectr. 2022 Oct 26;10(5):e0262022. doi: 10.1128/spectrum.02620-22. Epub 2022 Sep 1. Microbiol Spectr. 2022. PMID: 36047778 Free PMC article.
-
Antimicrobial Activity of Diffusible and Volatile Metabolites Emitted by Beauveria bassiana: Chemical Profile of Volatile Organic Compounds (VOCs) Using SPME-GC/MS Analysis.Plants (Basel). 2023 Aug 3;12(15):2854. doi: 10.3390/plants12152854. Plants (Basel). 2023. PMID: 37571008 Free PMC article.
-
The Elongator Subunit Elp3 Regulates Development, Stress Tolerance, Cell Cycle, and Virulence in the Entomopathogenic Fungus Beauveria bassiana.J Fungi (Basel). 2022 Aug 10;8(8):834. doi: 10.3390/jof8080834. J Fungi (Basel). 2022. PMID: 36012822 Free PMC article.
-
A life-and-death struggle: interaction of insects with entomopathogenic fungi across various infection stages.Front Immunol. 2024 Jan 8;14:1329843. doi: 10.3389/fimmu.2023.1329843. eCollection 2023. Front Immunol. 2024. PMID: 38259477 Free PMC article. Review.
-
Transcription Factors BbPacC and Bbmsn2 Jointly Regulate Oosporein Production in Beauveria bassiana.Microbiol Spectr. 2022 Dec 21;10(6):e0311822. doi: 10.1128/spectrum.03118-22. Epub 2022 Nov 23. Microbiol Spectr. 2022. PMID: 36416546 Free PMC article.
References
-
- Brakhage AA. Regulation of fungal secondary metabolism. Nat Rev Microbiol. 2013;11(1):21–32. - PubMed
-
- Wiemann P, Keller NP. Strategies for mining fungal natural products. J Ind Microbiol Biotechnol. 2014;41(2):301–313. - PubMed
-
- Nützmann H-W, Schroeckh V, Brakhage AA. Regulatory cross talk and microbial induction of fungal secondary metabolite gene clusters. In: David AH, editor. Methods in Enzymology. Vol 517. Academic; New York: 2012. pp. 325–341. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

