Enhancing VTA Cav1.3 L-type Ca2+ channel activity promotes cocaine and mood-related behaviors via overlapping AMPA receptor mechanisms in the nucleus accumbens
- PMID: 28194001
- PMCID: PMC5555837
- DOI: 10.1038/mp.2017.9
Enhancing VTA Cav1.3 L-type Ca2+ channel activity promotes cocaine and mood-related behaviors via overlapping AMPA receptor mechanisms in the nucleus accumbens
Abstract
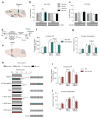
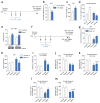
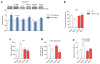
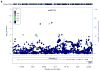
Genetic factors significantly influence susceptibility for substance abuse and mood disorders. Rodent studies have begun to elucidate a role of Cav1.3 L-type Ca2+ channels in neuropsychiatric-related behaviors, such as addictive and depressive-like behaviors. Human studies have also linked the CACNA1D gene, which codes for the Cav1.3 protein, with bipolar disorder. However, the neurocircuitry and the molecular mechanisms underlying the role of Cav1.3 in neuropsychiatric phenotypes are not well established. In the present study, we directly manipulated Cav1.3 channels in Cav1.2 dihydropyridine insensitive mutant mice and found that ventral tegmental area (VTA) Cav1.3 channels mediate cocaine-related and depressive-like behavior through a common nucleus accumbens (NAc) shell calcium-permeable α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (CP-AMPAR) mechanism that requires GluA1 phosphorylation at S831. Selective activation of VTA Cav1.3 with (±)-BayK-8644 (BayK) enhanced cocaine conditioned place preference and cocaine psychomotor activity while inducing depressive-like behavior, an effect not observed in S831A phospho-mutant mice. Infusion of the CP-AMPAR-specific blocker Naspm into the NAc shell reversed the cocaine and depressive-like phenotypes. In addition, activation of VTA Cav1.3 channels resulted in social behavioral deficits. In contrast to the cocaine- and depression-related phenotypes, GluA1/A2 AMPARs in the NAc core mediated social deficits, independent of S831-GluA1 phosphorylation. Using a candidate gene analysis approach, we also identified single-nucleotide polymorphisms in the CACNA1D gene associated with cocaine dependence in human subjects. Together, our findings reveal novel, overlapping mechanisms through which VTA Cav1.3 mediates cocaine-related, depressive-like and social phenotypes, suggesting that Cav1.3 may serve as a target for the treatment of neuropsychiatric symptoms.
Conflict of interest statement
None
Figures

References
-
- Kendler KS, Prescott CA, Myers J, Neale MC. The structure of genetic and environmental risk factors for common psychiatric and substance use disorders in men and women. Arch Gen Psychiatry. 2003;60(9):929–937. - PubMed
-
- Goldman D, Oroszi G, Ducci F. The genetics of addictions: uncovering the genes. Nat Rev Genet. 2005;6(7):521–532. - PubMed
MeSH terms
Substances
Grants and funding
- U01 HG004438/HG/NHGRI NIH HHS/United States
- U01 HG004446/HG/NHGRI NIH HHS/United States
- R01 DA029122/DA/NIDA NIH HHS/United States
- P01 CA089392/CA/NCI NIH HHS/United States
- R01 HL087120/HL/NHLBI NIH HHS/United States
- U01 HG004422/HG/NHGRI NIH HHS/United States
- R01 DA013423/DA/NIDA NIH HHS/United States
- R01 NS084190/NS/NINDS NIH HHS/United States
- U10 AA008401/AA/NIAAA NIH HHS/United States
- R01 DC009433/DC/NIDCD NIH HHS/United States
- R21 DA023686/DA/NIDA NIH HHS/United States
- HHSN268200782096C/HG/NHGRI NIH HHS/United States
- P 27809/FWF_/Austrian Science Fund FWF/Austria
- R01 AA022994/AA/NIAAA NIH HHS/United States
- R21 DA038048/DA/NIDA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

