Genetic Otx2 mis-localization delays critical period plasticity across brain regions
- PMID: 28194008
- PMCID: PMC5400722
- DOI: 10.1038/mp.2017.1
Genetic Otx2 mis-localization delays critical period plasticity across brain regions
Erratum in
-
Genetic Otx2 mis-localization delays critical period plasticity across brain regions.Mol Psychiatry. 2017 May;22(5):785. doi: 10.1038/mp.2017.83. Epub 2017 Apr 4. Mol Psychiatry. 2017. PMID: 28373687 No abstract available.
Abstract
Accumulation of non-cell autonomous Otx2 homeoprotein in postnatal mouse visual cortex (V1) has been implicated in both the onset and closure of critical period (CP) plasticity. Here, we show that a genetic point mutation in the glycosaminoglycan recognition motif of Otx2 broadly delays the maturation of pivotal parvalbumin-positive (PV+) interneurons not only in V1 but also in the primary auditory (A1) and medial prefrontal cortex (mPFC). Consequently, not only visual, but also auditory plasticity is delayed, including the experience-dependent expansion of tonotopic maps in A1 and the acquisition of acoustic preferences in mPFC, which mitigates anxious behavior. In addition, Otx2 mis-localization leads to dynamic turnover of selected perineuronal net (PNN) components well beyond the normal CP in V1 and mPFC. These findings reveal widespread actions of Otx2 signaling in the postnatal cortex controlling the maturational trajectory across modalities. Disrupted PV+ network function and deficits in PNN integrity are implicated in a variety of psychiatric illnesses, suggesting a potential global role for Otx2 function in establishing mental health.
Conflict of interest statement
The authors declare no financial interest related to this work.
Figures



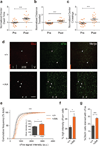
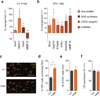
References
-
- Hensch TK. Critical period plasticity in local cortical circuits. Nature reviews Neuroscience. 2005;6(11):877–888. - PubMed
-
- Sanke RF. Amblyopia. American family physician. 1988;37(2):275–278. - PubMed
-
- Fagiolini M, Hensch TK. Inhibitory threshold for critical-period activation in primary visual cortex. Nature. 2000;404(6774):183–186. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

