Induced p53 loss in mouse luminal cells causes clonal expansion and development of mammary tumours
- PMID: 28194015
- PMCID: PMC5316831
- DOI: 10.1038/ncomms14431
Induced p53 loss in mouse luminal cells causes clonal expansion and development of mammary tumours
Abstract
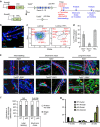
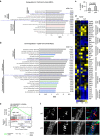
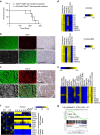
Most breast cancers may have a luminal origin. TP53 is one of the most frequently mutated genes in breast cancers. However, how p53 deficiency contributes to breast tumorigenesis from luminal cells remains elusive. Here we report that induced p53 loss in Krt8+ mammary luminal cells leads to their clonal expansion without directly affecting their luminal identity. All induced mice develop mammary tumours with 9qA1 (Yap1) and/or 6qA2 (Met) amplification(s). These tumours exhibit a mammary stem cell (MaSC)-like expression signature and most closely resemble claudin-low breast cancer. Thus, although p53 does not directly control the luminal fate, its loss facilitates acquisition of MaSC-like properties by luminal cells and predisposes them to development of mammary tumours with loss of luminal identity. Our data also suggest that claudin-low breast cancer can develop from luminal cells, possibly via a basal-like intermediate state, although further study using a different luminal promoter is needed to fully support this conclusion.
Conflict of interest statement
The authors declare no competing financial interests.
Figures



Similar articles
-
p53 controls the plasticity of mammary luminal progenitor cells downstream of Met signaling.Breast Cancer Res. 2019 Jan 25;21(1):13. doi: 10.1186/s13058-019-1101-8. Breast Cancer Res. 2019. PMID: 30683141 Free PMC article.
-
Targeted Pten deletion plus p53-R270H mutation in mouse mammary epithelium induces aggressive claudin-low and basal-like breast cancer.Breast Cancer Res. 2016 Jan 19;18(1):9. doi: 10.1186/s13058-015-0668-y. Breast Cancer Res. 2016. PMID: 26781438 Free PMC article.
-
Atypical cell cycle regulation promotes mammary stem cell expansion during mammary development and tumourigenesis.Breast Cancer Res. 2024 Jun 28;26(1):106. doi: 10.1186/s13058-024-01862-1. Breast Cancer Res. 2024. PMID: 38943151 Free PMC article.
-
Identification of cellular and genetic drivers of breast cancer heterogeneity in genetically engineered mouse tumour models.J Pathol. 2014 Jun;233(2):124-37. doi: 10.1002/path.4345. J Pathol. 2014. PMID: 24615332
-
Of mice and Myc: c-Myc and mammary tumorigenesis.J Mammary Gland Biol Neoplasia. 2004 Jan;9(1):27-37. doi: 10.1023/B:JOMG.0000023586.69263.0b. J Mammary Gland Biol Neoplasia. 2004. PMID: 15082916 Review.
Cited by
-
p53 shades of Hippo.Cell Death Differ. 2018 Jan;25(1):81-92. doi: 10.1038/cdd.2017.163. Epub 2017 Oct 6. Cell Death Differ. 2018. PMID: 28984872 Free PMC article. Review.
-
Cells-of-Origin of Breast Cancer and Intertumoral Heterogeneity.Adv Exp Med Biol. 2025;1464:151-165. doi: 10.1007/978-3-031-70875-6_9. Adv Exp Med Biol. 2025. PMID: 39821025 Review.
-
Tumor suppressor FRMD3 controls mammary epithelial cell fate determination via notch signaling pathway.Sci Adv. 2024 Jul 5;10(27):eadk8958. doi: 10.1126/sciadv.adk8958. Epub 2024 Jul 3. Sci Adv. 2024. PMID: 38959315 Free PMC article.
-
p53 controls the plasticity of mammary luminal progenitor cells downstream of Met signaling.Breast Cancer Res. 2019 Jan 25;21(1):13. doi: 10.1186/s13058-019-1101-8. Breast Cancer Res. 2019. PMID: 30683141 Free PMC article.
-
Research progress of Claudin-low breast cancer.Front Oncol. 2023 Oct 11;13:1226118. doi: 10.3389/fonc.2023.1226118. eCollection 2023. Front Oncol. 2023. PMID: 37904877 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

