Protective role of fructokinase blockade in the pathogenesis of acute kidney injury in mice
- PMID: 28194018
- PMCID: PMC5316807
- DOI: 10.1038/ncomms14181
Protective role of fructokinase blockade in the pathogenesis of acute kidney injury in mice
Abstract
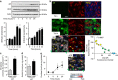
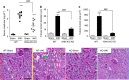
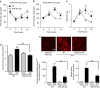
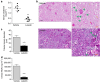
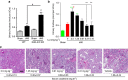
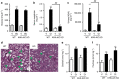
Acute kidney injury is associated with high mortality, especially in intensive care unit patients. The polyol pathway is a metabolic route able to convert glucose into fructose. Here we show the detrimental role of endogenous fructose production by the polyol pathway and its metabolism through fructokinase in the pathogenesis of ischaemic acute kidney injury (iAKI). Consistent with elevated urinary fructose in AKI patients, mice undergoing iAKI show significant polyol pathway activation in the kidney cortex characterized by high levels of aldose reductase, sorbitol and endogenous fructose. Wild type but not fructokinase knockout animals demonstrate severe kidney injury associated with ATP depletion, elevated uric acid, oxidative stress and inflammation. Interestingly, both the renal injury and dysfunction in wild-type mice undergoing iAKI is significantly ameliorated when exposed to luteolin, a recently discovered fructokinase inhibitor. This study demonstrates a role for fructokinase and endogenous fructose as mediators of acute renal disease.
Conflict of interest statement
R.J.J. has grants with Amway and Danone and is on the Scientific Advisory Board of Amway. R.J.J. also has authored two lay books on sugar (fructose) and its role in obesity and metabolic syndrome (The Sugar Fix, Rodale, 2008 and The Fat Switch, mercola.com, 2012). M.A.L., T.I. and R.J.J. have patents and patent application related to the blockade of fructokinase in the prevention and treatment of sugar-induced metabolic syndrome and renal disease. M.A.L., M.T.L., C.R.-J., M.F.W. and R.J.J. are members of Colorado Research Partners, LLC, a company focused on the development of fructokinase inhibitors. All other authors declare no competing financial interests.
Figures



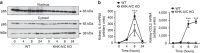
Comment in
-
Acute kidney injury: Benefits of blocking fructokinase.Nat Rev Nephrol. 2017 Apr;13(4):192. doi: 10.1038/nrneph.2017.23. Epub 2017 Feb 27. Nat Rev Nephrol. 2017. PMID: 28239174 No abstract available.
-
The sweetest thing: blocking fructose metabolism to prevent acute kidney injury?Kidney Int. 2017 May;91(5):998-1000. doi: 10.1016/j.kint.2017.03.004. Kidney Int. 2017. PMID: 28407885 Free PMC article.
References
-
- Star R. A. Treatment of acute renal failure. Kidney Int. 54, 1817–1831 (1998). - PubMed
-
- Chertow G. M., Burdick E., Honour M., Bonventre J. V. & Bates D. W. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J. Am. Soc. Nephrol. 16, 3365–3370 (2005). - PubMed
-
- Simmons E. M. et al.. Plasma cytokine levels predict mortality in patients with acute renal failure. Kidney Int. 65, 1357–1365 (2004). - PubMed
-
- Elahi M. M., Lim M. Y., Joseph R. N., Dhannapuneni R. R. & Spyt T. J. Early hemofiltration improves survival in post-cardiotomy patients with acute renal failure. Eur. J. Cardiothorac. Surg. 26, 1027–1031 (2004). - PubMed
-
- Liangos O. et al.. Epidemiology and outcomes of acute renal failure in hospitalized patients: a national survey. Clin. J. Am. Soc. Nephrol. 1, 43–51 (2006). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

