Tracking the virus-like particles of Macrobrachium rosenbergii nodavirus in insect cells
- PMID: 28194311
- PMCID: PMC5301976
- DOI: 10.7717/peerj.2947
Tracking the virus-like particles of Macrobrachium rosenbergii nodavirus in insect cells
Abstract
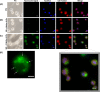
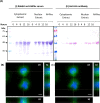
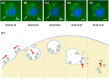
Macrobrachium rosenbergii nodavirus (MrNv) poses a major threat to the prawn industry. Currently, no effective vaccine and treatment are available to prevent the spread of MrNv. Its infection mechanism and localisation in a host cell are also not well characterised. The MrNv capsid protein (MrNvc) produced in Escherichia coli self-assembled into virus-like particles (VLPs) resembling the native virus. Thus, fluorescein labelled MrNvc VLPs were employed as a model to study the virus entry and localisation in Spodoptera frugiperda, Sf9 cells. Through fluorescence microscopy and sub-cellular fractionation, the MrNvc was shown to enter Sf9 cells, and eventually arrived at the nucleus. The presence of MrNvc within the cytoplasm and nucleus of Sf9 cells was further confirmed by the Z-stack imaging. The presence of ammonium chloride (NH4Cl), genistein, methyl-β-cyclodextrin or chlorpromazine (CPZ) inhibited the entry of MrNvc into Sf9 cells, but cytochalasin D did not inhibit this process. This suggests that the internalisation of MrNvc VLPs is facilitated by caveolae- and clathrin-mediated endocytosis. The whole internalisation process of MrNvc VLPs into a Sf9 cell was recorded with live cell imaging. We have also identified a potential nuclear localisation signal (NLS) of MrNvc through deletion mutagenesis and verified by classical-NLS mapping. Overall, this study provides an insight into the journey of MrNvc VLPs in insect cells.
Keywords: Endosome; Nodavirus; Nuclear translocation; Sub-cellular localisation; Virus-like particle.
Conflict of interest statement
The authors declare there are no competing interests.
Figures


References
LinkOut - more resources
Full Text Sources
Other Literature Sources

