Real-time genomic profiling of histiocytoses identifies early-kinase domain BRAF alterations while improving treatment outcomes
- PMID: 28194436
- PMCID: PMC5291734
- DOI: 10.1172/jci.insight.89473
Real-time genomic profiling of histiocytoses identifies early-kinase domain BRAF alterations while improving treatment outcomes
Abstract
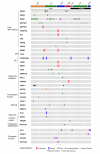
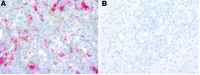
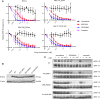
Many patients with histiocytic disorders such as Langerhans cell histiocytosis (LCH) or Erdheim-Chester disease (ECD) have treatment-refractory disease or suffer recurrences. Recent findings of gene mutations in histiocytoses have generated options for targeted therapies. We sought to determine the utility of prospective sequencing of select genes to further characterize mutations and identify targeted therapies for patients with histiocytoses. Biopsies of 72 patients with a variety of histiocytoses underwent comprehensive genomic profiling with targeted DNA and RNA sequencing. Fifteen patients (21%) carried the known BRAF V600E mutation, and 11 patients (15%) carried various mutations in MAP2K1, which we confirm induce constitutive activation of extracellular signal-regulated kinase (ERK) and were sensitive to inhibitors of mitogen-activated protein kinase kinase (MEK, the product of MAP2K1). We also identified recurring ALK rearrangements, and 4 LCH patients with an uncommon in-frame deletion in BRAF (N486_P490del or N486_T491>K), resulting in constitutive activation of ERK with resistance to V600E-specific inhibitors. We subsequently describe clinical cases where patients with aggressive multisystem LCH experience dramatic and sustained responses to monotherapy with either dabrafenib or trametinib. These findings support our conclusion that comprehensive genomic profiling should be regularly applied to these disorders at diagnosis, and can positively impact clinical care.
Conflict of interest statement
S. Ali, M. Bailey, P. Stephens, V.A. Miller, and J.S. Ross are employees of Foundation Medicine Inc.
Figures



References
-
- Grois N, et al. Central nervous system disease in Langerhans cell histiocytosis. J Pediatr. 2010;156(6):873–81, 881.e1. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

