Emergency Department-Initiated Buprenorphine for Opioid Dependence with Continuation in Primary Care: Outcomes During and After Intervention
- PMID: 28194688
- PMCID: PMC5442013
- DOI: 10.1007/s11606-017-3993-2
Emergency Department-Initiated Buprenorphine for Opioid Dependence with Continuation in Primary Care: Outcomes During and After Intervention
Abstract
Background: Emergency department (ED)-initiated buprenorphine/naloxone with continuation in primary care was found to increase engagement in addiction treatment and reduce illicit opioid use at 30 days compared to referral only or a brief intervention with referral.
Objective: To evaluate the long-term outcomes at 2, 6 and 12 months following ED interventions.
Design: Evaluation of treatment engagement, drug use, and HIV risk among a cohort of patients from a randomized trial who completed at least one long-term follow-up assessment.
Participants: A total of 290/329 patients (88% of the randomized sample) were included. The followed cohort did not differ significantly from the randomized sample.
Interventions: ED-initiated buprenorphine with 10-week continuation in primary care, referral, or brief intervention were provided in the ED at study entry.
Main measures: Self-reported engagement in formal addiction treatment, days of illicit opioid use, and HIV risk (2, 6, 12 months); urine toxicology (2, 6 months).
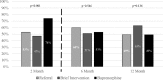
Key results: A greater number of patients in the buprenorphine group were engaged in addiction treatment at 2 months [68/92 (74%), 95% CI 65-83] compared with referral [42/79 (53%), 95% CI 42-64] and brief intervention [39/83 (47%), 95% CI 37-58; p < 0.001]. The differences were not significant at 6 months [51/92 (55%), 95% CI 45-65; 46/70 (66%) 95% CI 54-76; 43/76 (57%) 95% CI 45-67; p = 0.37] or 12 months [42/86 (49%) 95% CI 39-59; 37/73 (51%) 95% CI 39-62; 49/78 (63%) 95% CI 52-73; p = 0.16]. At 2 months, the buprenorphine group reported fewer days of illicit opioid use [1.1 (95% CI 0.6-1.6)] versus referral [1.8 (95% CI 1.2-2.3)] and brief intervention [2.0 (95% CI 1.5-2.6), p = 0.04]. No significant differences in illicit opioid use were observed at 6 or 12 months. There were no significant differences in HIV risk or rates of opioid-negative urine results at any time.
Conclusions: ED-initiated buprenorphine was associated with increased engagement in addiction treatment and reduced illicit opioid use during the 2-month interval when buprenorphine was continued in primary care. Outcomes at 6 and 12 months were comparable across all groups.
Keywords: emergency medicine; opioid use disorder; primary care; substance use disorder.
Conflict of interest statement
Conflict of Interest
Dr. Fiellin has received honoraria from Pinney Associates for serving on an external advisory board monitoring the diversion and abuse of buprenorphine. The remaining authors have no conflicts.
Funders
The study was supported by grants 5R01DA025991 and K12DA033312 from the National Institute on Drug Abuse (NIDA), and Reckitt Benckiser Pharmaceuticals provided buprenorphine through NIDA. The funding organization had no role in the design or conduct of the study; collection, management, analysis or interpretation of the data; preparation, review or approval of the manuscript; or the decision to submit the manuscript for publication.
Prior Presentation
The College on Problems of Drug Dependence, June 2015, Phoenix, Arizona.
Figures

Comment in
-
Capsule Commentary on D'Onofrio et al., Emergency Department-Initiated Buprenorphine for Opioid Dependence with Continuation in Primary Care: Outcomes During and After Intervention.J Gen Intern Med. 2017 Jun;32(6):683. doi: 10.1007/s11606-017-4015-0. J Gen Intern Med. 2017. PMID: 28243878 Free PMC article. No abstract available.
References
-
- Centers for Disease Control, Prevention Emergency department visits involving nonmedical use of selected prescription drugs - United States, 2004–2008. MMWR Morb Mortal Wkly Rep. 2010;59(23):705–9. - PubMed
-
- Centers for Disease Control, Prevention CDC grand rounds: prescription drug overdoses - a U.S. epidemic. MMWR Morb Mortal Wkly Rep. 2012;61(1):10–3. - PubMed
-
- Surgeon General Report: U.S. Department of Health and Human Services (HHS), Office of the Surgeon General, Facing Addiction in America: The Surgeon General’s Report on Alcohol, Drugs, and Health, Executive Summary. Washington, DC: HHS, November 2016. - PubMed
-
- Gowing L, Farrell MF, Bornemann R, Sullivan LE, Ali R. Oral substitution treatment of injecting opioid users for prevention of HIV infection. Cochrane Database Syst Rev. 2011;8:CD004145. - PubMed
-
- Anonymous . Guidelines for the psychosocially assisted pharmacological treatment of opioid dependence. Geneva: World Health Organization; 2009. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

