Fetal adaptations in insulin secretion result from high catecholamines during placental insufficiency
- PMID: 28194805
- PMCID: PMC5538202
- DOI: 10.1113/JP273324
Fetal adaptations in insulin secretion result from high catecholamines during placental insufficiency
Abstract
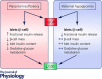
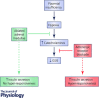
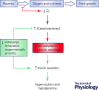
Placental insufficiency and intrauterine growth restriction (IUGR) of the fetus affects approximately 8% of all pregnancies and is associated with short- and long-term disturbances in metabolism. In pregnant sheep, experimental models with a small, defective placenta that restricts delivery of nutrients and oxygen to the fetus result in IUGR. Low blood oxygen concentrations increase fetal plasma catecholamine concentrations, which lower fetal insulin concentrations. All of these observations in sheep models with placental insufficiency are consistent with cases of human IUGR. We propose that sustained high catecholamine concentrations observed in the IUGR fetus produce developmental adaptations in pancreatic β-cells that impair fetal insulin secretion. Experimental evidence supporting this hypothesis shows that chronic elevation in circulating catecholamines in IUGR fetuses persistently inhibits insulin concentrations and secretion. Elevated catecholamines also allow for maintenance of a normal fetal basal metabolic rate despite low fetal insulin and glucose concentrations while suppressing fetal growth. Importantly, a compensatory augmentation in insulin secretion occurs following inhibition or cessation of catecholamine signalling in IUGR fetuses. This finding has been replicated in normally grown sheep fetuses following a 7-day noradrenaline (norepinephrine) infusion. Together, these programmed effects will potentially create an imbalance between insulin secretion and insulin-stimulated glucose utilization in the neonate which probably explains the transient hyperinsulinism and hypoglycaemia in some IUGR infants.
Keywords: developmental programming; epinephrine; intrauterine growth restriction; norepinephrine; β-cell.
© 2017 The Authors. The Journal of Physiology © 2017 The Physiological Society.
Figures
Comment in
-
Stress during pregnancy and its life-long consequences for the infant.J Physiol. 2017 Aug 1;595(15):5055-5056. doi: 10.1113/JP274444. J Physiol. 2017. PMID: 28762512 Free PMC article. No abstract available.
References
-
- Barker DJ, Hales CN, Fall CH, Osmond C, Phipps K & Clark PM (1993). Type 2 (non‐insulin‐dependent) diabetes mellitus, hypertension and hyperlipidaemia (syndrome X): relation to reduced fetal growth. Diabetologia 36, 62–67. - PubMed
-
- Bassett JM & Hanson C (1998). Catecholamines inhibit growth in fetal sheep in the absence of hypoxemia. Am J Physiol 274, R1536–R1545. - PubMed
-
- Bazaes RA, Salazar TE, Pittaluga E, Pena V, Alegria A, Iniguez G, Ong KK, Dunger DB & Mericq MV (2003). Glucose and lipid metabolism in small for gestational age infants at 48 hours of age. Pediatrics 111, 804–809. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

