Spongy Gels by a Top-Down Approach from Polymer Fibrous Sponges
- PMID: 28194915
- PMCID: PMC5363351
- DOI: 10.1002/anie.201611787
Spongy Gels by a Top-Down Approach from Polymer Fibrous Sponges
Erratum in
-
Corrigendum: Spongy Gels by a Top-Down Approach from Polymer Fibrous Sponges.Angew Chem Int Ed Engl. 2017 May 15;56(21):5652. doi: 10.1002/anie.201703533. Angew Chem Int Ed Engl. 2017. PMID: 28481457 Free PMC article. No abstract available.
Abstract
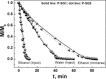
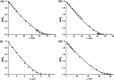
Ultralight cellular sponges offer a unique set of properties. We show here that solvent uptake by these sponges results in new gel-like materials, which we term spongy gels. The appearance of the spongy gels is very similar to classic organogels. Usually, organogels are formed by a bottom-up process. In contrast, the spongy gels are formed by a top-down approach that offers numerous advantages for the design of their properties, reproducibility, and stability. The sponges themselves represent the scaffold of a gel that could be filled with a solvent, and thereby form a mechanically stable gel-like material. The spongy gels are independent of a time-consuming or otherwise demanding in situ scaffold formation. As solvent evaporation from gels is a concern for various applications, we also studied solvent evaporation of wetting and non-wetting liquids dispersed in the sponge.
Keywords: electrospinning; gels; sponges.
© 2017 The Authors. Published by Wiley-VCH Verlag GmbH & Co. KGaA.
Figures




References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

