GSK-3β Inhibition Induced Neuroprotection, Regeneration, and Functional Recovery After Intracerebral Hemorrhagic Stroke
- PMID: 28195036
- PMCID: PMC5657706
- DOI: 10.3727/096368916X694364
GSK-3β Inhibition Induced Neuroprotection, Regeneration, and Functional Recovery After Intracerebral Hemorrhagic Stroke
Abstract
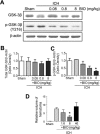
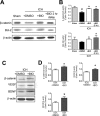
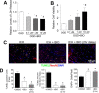
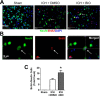
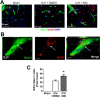
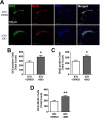
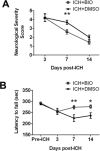
Hemorrhagic stroke is a devastating disease that lacks effective therapies. In the present investigation, we tested 6-bromoindirubin-3'-oxime (BIO) as a selective glycogen synthase kinase-3β (GSK-3β) inhibitor in a mouse model of intracerebral hemorrhage (ICH). ICH was induced by injection of collagenase IV into the striatum of 8- to 10-week-old C57BL/6 mice. BIO (8 μg/kg, IP) was administered following either an acute delivery (0-2 h delay) or a prolonged regimen (every 48 h starting at 3 days post-ICH). At 2 days post-ICH, the acute BIO treatment significantly reduced the hematoma volume. In the perihematoma regions, BIO administration blocked GSK-3β phosphorylation/activation, increased Bcl-2 and β-catenin levels, and significantly increased viability of neurons and other cell types. The prolonged BIO regimen maintained a higher level of β-catenin, upregulated VEGF and BDNF, and promoted neurogenesis and angiogenesis in peri-injury zones at 14 days after ICH. The BIO treatment also promoted proliferation of neural stem cells (NSCs) and migration of nascent DCX+ neuroblasts from the subventricular zone (SVZ) to the lesioned cortex. BIO improved functional outcomes on both the neurological severity score and rotarod tests. The findings of this study corroborate the neuroprotective and regenerative effects of BIO and suggest that the Wnt/GSK-3β/β-catenin pathway may be explored for the treatment of acute or chronic ICH.
Figures
Similar articles
-
[Gly14]-Humanin offers neuroprotection through glycogen synthase kinase-3β inhibition in a mouse model of intracerebral hemorrhage.Behav Brain Res. 2013 Jun 15;247:132-9. doi: 10.1016/j.bbr.2013.03.023. Epub 2013 Mar 26. Behav Brain Res. 2013. PMID: 23538063
-
Delayed treatment of 6-Bromoindirubin-3'-oxime stimulates neurogenesis and functional recovery after focal ischemic stroke in mice.Int J Dev Neurosci. 2017 Apr;57:77-84. doi: 10.1016/j.ijdevneu.2017.01.002. Epub 2017 Jan 19. Int J Dev Neurosci. 2017. PMID: 28111255 Free PMC article.
-
Lithium posttreatment confers neuroprotection through glycogen synthase kinase-3β inhibition in intracerebral hemorrhage rats.J Neurosurg. 2017 Oct;127(4):716-724. doi: 10.3171/2016.7.JNS152995. Epub 2016 Oct 14. J Neurosurg. 2017. PMID: 27739937
-
Psychopharmacology of atypical antipsychotic drugs: From the receptor binding profile to neuroprotection and neurogenesis.Psychiatry Clin Neurosci. 2015 May;69(5):243-58. doi: 10.1111/pcn.12242. Epub 2014 Nov 6. Psychiatry Clin Neurosci. 2015. PMID: 25296946 Review.
-
Statins for neuroprotection in spontaneous intracerebral hemorrhage.Neurology. 2019 Dec 10;93(24):1056-1066. doi: 10.1212/WNL.0000000000008627. Epub 2019 Nov 11. Neurology. 2019. PMID: 31712367 Free PMC article. Review.
Cited by
-
Glycogen Synthase Kinase-3 Inhibitors: Preclinical and Clinical Focus on CNS-A Decade Onward.Front Mol Neurosci. 2022 Jan 21;14:792364. doi: 10.3389/fnmol.2021.792364. eCollection 2021. Front Mol Neurosci. 2022. PMID: 35126052 Free PMC article. Review.
-
Priming of the Cells: Hypoxic Preconditioning for Stem Cell Therapy.Chin Med J (Engl). 2017 Oct 5;130(19):2361-2374. doi: 10.4103/0366-6999.215324. Chin Med J (Engl). 2017. PMID: 28937044 Free PMC article. Review.
-
Glia and Neural Stem and Progenitor Cells of the Healthy and Ischemic Brain: The Workplace for the Wnt Signaling Pathway.Genes (Basel). 2020 Jul 16;11(7):804. doi: 10.3390/genes11070804. Genes (Basel). 2020. PMID: 32708801 Free PMC article. Review.
-
Competing Endogenous RNA and Coexpression Network Analysis for Identification of Potential Biomarkers and Therapeutics in association with Metastasis Risk and Progression of Prostate Cancer.Oxid Med Cell Longev. 2019 Aug 5;2019:8265958. doi: 10.1155/2019/8265958. eCollection 2019. Oxid Med Cell Longev. 2019. PMID: 31467637 Free PMC article.
-
A GLP1 receptor agonist diabetes drug ameliorates neurodegeneration in a mouse model of infantile neurometabolic disease.Sci Rep. 2022 Aug 15;12(1):13825. doi: 10.1038/s41598-022-17338-1. Sci Rep. 2022. PMID: 35970890 Free PMC article.
References
-
- Rosamond W, Flegal K, Furie K, Go A, Greenlund K, Haase N, Hailpern SM, Ho M, Howard V, Kissela B, Kittner S, Lloyd-Jones D, McDermott M, Meigs J, Moy C, Nichol G, O'Donnell C, Roger V, Sorlie P, Steinberger J, Thom T, Wilson M, Hong Y; and for the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics–-2008 update: A report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation 2008; 117(4): e25–146. - PubMed
-
- Sacco S, Marini C, Toni D, Olivieri L, Carolei A. Incidence and 10-year survival of intracerebral hemorrhage in a population-based registry. Stroke 2009; 40(2): 394–9. - PubMed
-
- van Asch CJ, Luitse MJ, Rinkel GJ, van der Tweel I, Algra A, Klijn CJ,. Incidence, case fatality, and functional outcome of intracerebral haemorrhage over time, according to age, sex, and ethnic origin: A systematic review and meta-analysis. Lancet Neurol. 2010; 9(2): 167–76. - PubMed
-
- Iihara K, Nishimura K, Kada A, Nakagawara J, Ogasawara K, Ono J, Shiokawa Y, Aruga T, Miyachi S, Nagata I, Toyoda K, Matsuda S, Miyamoto Y, Suzuki A, Ishikawa KB, Kataoka H, Nakamura F, Kamitani S. Effects of comprehensive stroke care capabilities on in-hospital mortality of patients with ischemic and hemorrhagic stroke: J-aspect study. PLoS One 2014; 9(5): e96819. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

