Speed and segmentation control mechanisms characterized in rhythmically-active circuits created from spinal neurons produced from genetically-tagged embryonic stem cells
- PMID: 28195039
- PMCID: PMC5308898
- DOI: 10.7554/eLife.21540
Speed and segmentation control mechanisms characterized in rhythmically-active circuits created from spinal neurons produced from genetically-tagged embryonic stem cells
Abstract
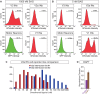
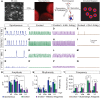
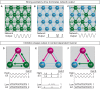
Flexible neural networks, such as the interconnected spinal neurons that control distinct motor actions, can switch their activity to produce different behaviors. Both excitatory (E) and inhibitory (I) spinal neurons are necessary for motor behavior, but the influence of recruiting different ratios of E-to-I cells remains unclear. We constructed synthetic microphysical neural networks, called circuitoids, using precise combinations of spinal neuron subtypes derived from mouse stem cells. Circuitoids of purified excitatory interneurons were sufficient to generate oscillatory bursts with properties similar to in vivo central pattern generators. Inhibitory V1 neurons provided dual layers of regulation within excitatory rhythmogenic networks - they increased the rhythmic burst frequency of excitatory V3 neurons, and segmented excitatory motor neuron activity into sub-networks. Accordingly, the speed and pattern of spinal circuits that underlie complex motor behaviors may be regulated by quantitatively gating the intra-network cellular activity ratio of E-to-I neurons.
Keywords: cell biology; circuitoid; embryonic stem cells; excitatory-inhibitory balance; mouse; neuroscience; rhythmicity; synthetic network.
Conflict of interest statement
The authors declare that no competing interests exist.
Figures













Similar articles
-
Excitatory components of the mammalian locomotor CPG.Brain Res Rev. 2008 Jan;57(1):56-63. doi: 10.1016/j.brainresrev.2007.07.002. Epub 2007 Nov 7. Brain Res Rev. 2008. PMID: 17988744 Review.
-
Functional Interactions between Mammalian Respiratory Rhythmogenic and Premotor Circuitry.J Neurosci. 2016 Jul 6;36(27):7223-33. doi: 10.1523/JNEUROSCI.0296-16.2016. J Neurosci. 2016. PMID: 27383596 Free PMC article.
-
Origin and circuitry of spinal locomotor interneurons generating different speeds.Curr Opin Neurobiol. 2018 Dec;53:16-21. doi: 10.1016/j.conb.2018.04.024. Epub 2018 May 4. Curr Opin Neurobiol. 2018. PMID: 29733915 Review.
-
Separate microcircuit modules of distinct v2a interneurons and motoneurons control the speed of locomotion.Neuron. 2014 Aug 20;83(4):934-43. doi: 10.1016/j.neuron.2014.07.018. Epub 2014 Aug 7. Neuron. 2014. PMID: 25123308
-
Delineating the Diversity of Spinal Interneurons in Locomotor Circuits.J Neurosci. 2017 Nov 8;37(45):10835-10841. doi: 10.1523/JNEUROSCI.1829-17.2017. J Neurosci. 2017. PMID: 29118212 Free PMC article. Review.
Cited by
-
Subtype Diversification and Synaptic Specificity of Stem Cell-Derived Spinal Interneurons.Neuron. 2018 Oct 10;100(1):135-149.e7. doi: 10.1016/j.neuron.2018.09.016. Neuron. 2018. PMID: 30308166 Free PMC article.
-
In Vitro Modelling of Nerve-Muscle Connectivity in a Compartmentalised Tissue Culture Device.Adv Biosyst. 2019 Jul;3(7):1800307. doi: 10.1002/adbi.201800307. Epub 2019 May 8. Adv Biosyst. 2019. PMID: 31428672 Free PMC article.
-
Transplantation of Neural Progenitors and V2a Interneurons after Spinal Cord Injury.J Neurotrauma. 2018 Dec 15;35(24):2883-2903. doi: 10.1089/neu.2017.5439. Epub 2018 Aug 10. J Neurotrauma. 2018. PMID: 29873284 Free PMC article.
-
Hindbrain V2a Neurons Pattern Rhythmic Activity of Motor Neurons in a Reticulospinal Coculture.Front Neurosci. 2019 Oct 17;13:1077. doi: 10.3389/fnins.2019.01077. eCollection 2019. Front Neurosci. 2019. PMID: 31680817 Free PMC article.
-
Intermediate gray matter interneurons in the lumbar spinal cord play a critical and necessary role in coordinated locomotion.PLoS One. 2023 Oct 31;18(10):e0291740. doi: 10.1371/journal.pone.0291740. eCollection 2023. PLoS One. 2023. PMID: 37906544 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

