Patient acceptability and satisfaction with a low-dose solubilized vaginal estradiol softgel capsule, TX-004HR
- PMID: 28195995
- PMCID: PMC5526433
- DOI: 10.1097/GME.0000000000000848
Patient acceptability and satisfaction with a low-dose solubilized vaginal estradiol softgel capsule, TX-004HR
Abstract
Objective: TX-004HR is an investigational, muco-adhesive, vaginal, softgel capsule containing low-dose, solubilized, 17β-estradiol designed to treat postmenopausal vulvar and vaginal atrophy (VVA) and improve user experience without an applicator and less mess.
Methods: As part of the 12-week, placebo-controlled, double-blind, phase 3 REJOICE trial evaluating the efficacy/safety of 4-, 10-, and 25-μg TX-004HR in 764 postmenopausal women with VVA, a five-question product survey was administered. Pearson correlation coefficients were used to evaluate correlations between clinical endpoints (vaginal physiology, dyspareunia, and vaginal dryness) and patient acceptability and satisfaction.
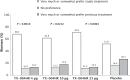
Results: Majority of the women receiving TX-004HR or placebo reported that the product was easy to use (85.4%-92.1%) and rated ease of capsule insertion as "good" to "excellent" (75.0%-82.6%). A significantly greater percentage of women reported being "very satisfied" or "satisfied" with TX-004HR (68.6%-76.3%) than with placebo (56.8%, P < 0.05 for all). A greater percentage of women "somewhat" or "very much" preferred TX-004HR over their previous treatment versus those taking placebo (P < 0.05). Significantly more women receiving TX-004HR (72.8%-80.5%) versus placebo (62.5%, P < 0.05) would "probably" or "definitely" consider using the product again. Dyspareunia and vaginal dryness reductions were correlated with higher product satisfaction and the percentage of women who would consider re-using TX-004HR.
Conclusions: TX-004HR had a high level of product acceptability, and more women were satisfied with TX-004HR, preferred it over their previous treatment, and would consider using it again versus placebo. Women may find TX-004HR to be a more acceptable product than current options to treat their dyspareunia associated with postmenopausal VVA.
Figures
References
-
- Lev-Sagie A. Vulvar and vaginal atrophy: physiology, clinical presentation, and treatment considerations. Clin Obstet Gynecol 2015; 58:476–491. - PubMed
-
- Portman DJ, Gass ML. Genitourinary syndrome of menopause: new terminology for vulvovaginal atrophy from the International Society for the Study of Women's Sexual Health and the North American Menopause Society. Menopause 2014; 21:1063–1068. - PubMed
-
- North American Menopause Society. Management of symptomatic vulvovaginal atrophy: 2013 position statement of The North American Menopause Society. Menopause 2013; 20:888–902. - PubMed
-
- Alexander NJ, Baker E, Kaptein M, Karck U, Miller L, Zampaglione E. Why consider vaginal drug administration? Fertil Steril 2004; 82:1–12. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials