Antiviral Activity, Safety, and Pharmacokinetics of Bictegravir as 10-Day Monotherapy in HIV-1-Infected Adults
- PMID: 28196003
- PMCID: PMC5389589
- DOI: 10.1097/QAI.0000000000001306
Antiviral Activity, Safety, and Pharmacokinetics of Bictegravir as 10-Day Monotherapy in HIV-1-Infected Adults
Abstract
Objective: To evaluate antiviral activity, safety, and pharmacokinetics of short-term monotherapy with bictegravir (BIC), a novel, potent HIV integrase strand transfer inhibitor (INSTI).
Design: Phase 1b, randomized, double-blinded, adaptive, sequential cohort, placebo-controlled study.
Methods: HIV-infected adults not taking antiretroviral therapy were randomized to receive BIC (5, 25, 50, or 100 mg) or placebo once daily for 10 days. Primary endpoint was time-weighted average change from baseline to day 11 (DAVG11) for plasma HIV-1 RNA. HIV-1 RNA, adverse events (AEs), and laboratory assessments were evaluated through day 17.
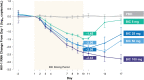
Results: Twenty participants were enrolled (n = 4/group). Mean DAVG11 ranged from -0.92 to -1.61 across BIC doses versus -0.01 for placebo. Significant reductions in plasma HIV-1 RNA from baseline at day 11 were observed for all BIC doses compared with placebo (P < 0.001); mean decreases were 1.45-2.43 log10 copies/mL. Increased BIC exposures correlated with increased reduction in plasma HIV-1 RNA from baseline on day 11. Three participants on BIC (50 or 100 mg) achieved plasma HIV-1 RNA <50 copies/mL by end of study. Median Tmax ranged from 1.0 to 1.8 hours (day 1, postdose) and 1.3-2.7 hours (day 10), with median t1/2 ranging from 15.9 to 20.9 hours. No participant developed primary INSTI-R substitution through day 17. BIC was well tolerated, with no discontinuations because of adverse events.
Conclusions: BIC is a novel, potent, unboosted INSTI that demonstrated rapid, dose-dependent declines in HIV-1 RNA after 10 days of monotherapy. BIC was well tolerated, and displayed rapid absorption and a half-life supportive of once-daily therapy in HIV-infected subjects.
Conflict of interest statement
X.W., H.Z., K.W., A.C., E.Q., and H.M., are employees of Gilead and hold stock interest in the company. The remaining authors have no conflicts of interest to disclose.
Figures
References
-
- Adolescents PoAGfAa. Guidelines for the Use of Antiretroviral Agents in HIV-1 Infected Adults and Adolescents. Services DoHaH; 2016. Available at: http://aidsinfo.nih.gov/contentfiles/lvguidelines/AdultandAdolescentGL.pdf. Accessed 27 July, 2016.
-
- Worm SW, Sabin C, Weber R, et al. Risk of myocardial infarction in patients with HIV infection exposed to specific individual antiretroviral drugs from the 3 major drug classes: the data collection on adverse events of anti-HIV drugs (D: A:D) study. J Infect Dis. 2010;201:318–330. - PubMed
-
- Young J, Xiao Y, Moodie EE, et al. Effect of cumulating exposure to abacavir on the risk of cardiovascular disease events in patients from the Swiss HIV cohort study. J Acquir Immune Defic Syndr. 2015;69:413–421. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical