Long-term results and recurrence patterns from SCOPE-1: a phase II/III randomised trial of definitive chemoradiotherapy +/- cetuximab in oesophageal cancer
- PMID: 28196063
- PMCID: PMC5355926
- DOI: 10.1038/bjc.2017.21
Long-term results and recurrence patterns from SCOPE-1: a phase II/III randomised trial of definitive chemoradiotherapy +/- cetuximab in oesophageal cancer
Abstract
Background: The SCOPE-1 study tested the role of adding cetuximab to conventional definitive chemoradiotherapy (dCRT), and demonstrated greater toxicity and worse survival outcomes. We present the long-term outcomes and patterns of recurrence.
Methods: SCOPE-1 was a phase II/III trial in which patients were randomised to cisplatin 60 mg m-2 (day 1) and capecitabine 625 mg m-2 bd (days 1-21) for four cycles +/- cetuximab 400 mg m-2 day 1 then by 250 mg m-2 weekly. Radiotherapy consisted of 50 Gy/25# given concurrently with cycles 3 and 4. Recruitment was between February 2008 and February 2012, when the IDMC recommended closure on the basis of futility.
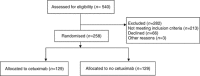
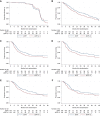
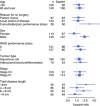
Results: About 258 patients (dCRT=129; dCRT+cetuximab (dCRT+C)=129) were recruited from 36 centres. About 72.9% (n=188) had squamous cell histology. The median follow-up (IQR) was 46.2 (35.9-48.3) months for surviving patients. The median overall survival (OS; months; 95% CI) was 34.5 (24.7-42.3) in dCRT and 24.7 (18.6-31.3) in dCRT+C (hazard ratio (HR)=1.25, 95% CIs: 0.93-1.69, P=0.137). Median progression-free survival (PFS; months; 95% CI) was 24.1 (15.3-29.9) and 15.9 (10.7-20.8) months, respectively (HR=1.28, 95% CIs: 0.94-1.75; P=0.114). On multivariable analysis only earlier stage, full-dose RT, and higher cisplatin dose intensity were associated with improved OS.
Conclusions: The mature analysis demonstrates that the dCRT regimen used in the study provided useful survival outcomes despite its use in patients who were largely unfit for surgery or who had inoperable disease. Given the competing risk of systemic and local failure, future studies should continue to focus on enhancing local control as well as optimising systemic therapy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Akimoto T, Hunter NR, Buchmiller L, Mason K, Ang KK, Milas L (1999) Inverse relationship between epidermal growth factor receptor expression and radiocurability of murine carcinomas. Clin Cancer Res 5(10): 2884–2890. - PubMed
-
- Ang KK, Zhang Q, Rosenthal DI, Nguyen-Tan PF, Sherman EJ, Weber RS, Galvin JM, Bonner JA, Harris J, El-Naggar AK, Gillison ML, Jordan RC, Konski AA, Thorstad WL, Trotti A, Beitler JJ, Garden AS, Spanos WJ, Yom SS, Axelrod RS (2014) Randomized phase III trial of concurrent accelerated radiation plus cisplatin with or without cetuximab for stage III to IV head and neck carcinoma: RTOG 0522. J Clin Oncol 32(27): 2940–2950. - PMC - PubMed
-
- Becerra CR, Hanna N, McCollum AD, Becharm N, Timmerman RD, DiMaio M, Kesler KA, Yu M, Yan T, Choy H (2013) A phase II study with cetuximab and radiation therapy for patients with surgically resectable esophageal and GE junction carcinomas: Hoosier Oncology Group G05-92. J Thorac Oncol 8(11): 1425–1429. - PubMed
-
- Bedenne L, Michel P, Bouche O, Milan C, Mariette C, Conroy T, Pezet D, Roullet B, Seitz JF, Herr JP, Paillot B, Arveux P, Bonnetain F, Binquet C (2007) Chemoradiation followed by surgery compared with chemoradiation alone in squamous cancer of the esophagus: FFCD 9102. J Clin Oncol 25(10): 1160–1168. - PubMed
-
- Bonner JA, Harari PM, Giralt J, Azarnia N, Shin DM, Cohen RB, Jones CU, Sur R, Raben D, Jassem J, Ove R, Kies MS, Baselga J, Youssoufian H, Amellal N, Rowinsky EK, Ang KK (2006) Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med 354(6): 567–578. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

