Alterations in estrogen signalling pathways upon acquisition of anthracycline resistance in breast tumor cells
- PMID: 28196134
- PMCID: PMC5308870
- DOI: 10.1371/journal.pone.0172244
Alterations in estrogen signalling pathways upon acquisition of anthracycline resistance in breast tumor cells
Abstract
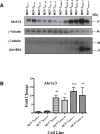
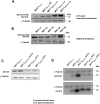
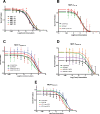
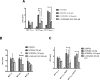
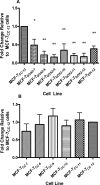
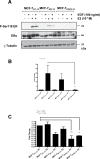
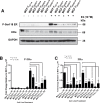
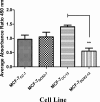
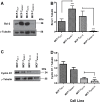
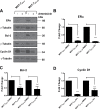
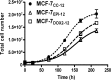
Intrinsic or acquired drug resistance is a major impediment to the successful treatment of women with breast cancer using chemotherapy. We have observed that MCF-7 breast tumor cells selected for resistance to doxorubicin or epirubicin (MCF-7DOX2 and MCF-7EPI cells, respectively) exhibited increased expression of several members of the aldo-keto reductase (AKR) gene family (in particular AKR1C3 and AKR1B10) relative to control MCF-7CC cells selected by propagation in the absence of drug. Normal cellular roles for the AKRs include the promotion of estrogen (E2) synthesis from estrone (E1) and the hydroxylation and detoxification of exogenous xenobiotics such as anthracycline chemotherapy drugs. While hydroxylation of anthracyclines strongly attenuates their cytotoxicity, it is unclear whether the enhanced AKR expression in the above anthracycline-resistant cells promotes E2 synthesis and/or alterations in E2 signalling pathways and whether such changes contribute to enhanced survival and anthracycline resistance. To determine the role of AKRs and E2 pathways in doxorubicin resistance, we examined changes in the expression of E2-related genes and proteins upon acquisition of doxorubicin resistance. We also assessed the effects of AKR overexpression or downregulation or the effects of activators or inhibitors of E2-dependent pathways on previously acquired resistance to doxorubicin. In this study we observed that the enhanced AKR expression upon acquisition of anthracycline resistance was, in fact, associated with enhanced E2 production. However, the expression of estrogen receptor α (ERα) was reduced by 2- to 5-fold at the gene transcript level and 2- to 20-fold at the protein level upon acquisition of anthracycline resistance. This was accompanied by an even stronger reduction in ERα phosphorylation and activity, including highly suppressed expression of two proteins under E2-dependent control (Bcl-2 and cyclin D1). The diminished Bcl-2 and cyclin D1 expression would be expected to reduce the growth rate of the cells, a hypothesis which was confirmed in subsequent cell proliferation experiments. AKR1C3 or AKR1B10 overexpression alone had no effect on doxorubicin sensitivity in MCF-7CC cells, while siRNA-mediated knockdown of AKR1C3 and/or AKR1B10 expression had no significant effect on sensitivity to doxorubicin in MCF-7DOX2 or MCF-7EPI cells. This suggested that enhanced or reduced AKR expression/activity is insufficient to confer anthracycline resistance or sensitivity to breast tumor cells, respectively. Rather, it would appear that AKR overexpression acts in concert with other proteins to confer anthracycline resistance, including reduced E2-dependent expression of both an important apoptosis inhibitor (Bcl-2) and a key protein associated with activation of cell cycle-dependent kinases (cyclin D1).
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
-
- Wilson RG, Kalonaros V, King M, Lockwood R, McNeill M. Comparative inhibition of nuclear RNA synthesis in cultured mouse leukemia L1210 cells by adriamycin and 4'-epi-adriamycin. Chem Biol Interact. 1981;37(3):351–63. - PubMed
-
- Komiyama T, Oki T, Inui T. Interaction of new anthracycline antibiotics with DNA. Effects on nucleic acid synthesis and binding to DNA. Biochim Biophys Acta. 1983;740(1):80–7. - PubMed
-
- Beck WT, Danks MK. Mechanisms of resistance to drugs that inhibit DNA topoisomerases. Semin Cancer Biol. 1991;2(4):235–44. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

