Mammalian enamel maturation: Crystallographic changes prior to tooth eruption
- PMID: 28196135
- PMCID: PMC5308864
- DOI: 10.1371/journal.pone.0171424
Mammalian enamel maturation: Crystallographic changes prior to tooth eruption
Abstract
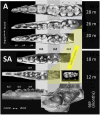
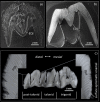
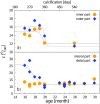
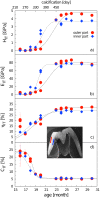
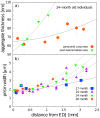
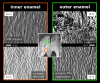
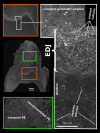
Using the distal molar of a minipig as a model, we studied changes in the microstructural characteristics of apatite crystallites during enamel maturation (16-23 months of postnatal age), and their effects upon the mechanical properties of the enamel coat. The slow rate of tooth development in a pig model enabled us to reveal essential heterochronies in particular components of the maturation process. The maturation changes began along the enamel-dentine junction (EDJ) of the trigonid, spreading subsequently to the outer layers of the enamel coat to appear at the surface zone with a 2-month delay. Correspondingly, at the distal part of the tooth the timing of maturation processes is delayed by 3-5 month compared to the mesial part of the tooth. The early stage of enamel maturation (16-20 months), when the enamel coat is composed almost exclusively of radial prismatic enamel, is characterized by a gradual increase in crystallite thickness (by a mean monthly increment of 3.8 nm); and an increase in the prism width and thickness of crystals composed of elementary crystallites. The late stage of maturation (the last two months prior to tooth eruption), marked with the rapid appearance of the interprismatic matrix (IPM) during which the crystals densely infill spaces between prisms, is characterized by an abrupt decrease in microstrain and abrupt changes in the micromechanical properties of the enamel: a rapid increase in its ability to resist long-term load and its considerable hardening. The results suggest that in terms of crystallization dynamics the processes characterizing the early and late stage of mammalian enamel maturation represent distinct entities. In regards to common features with enamel formation in the tribosphenic molar we argue that the separation of these processes could be a common apomorphy of mammalian amelogenetic dynamics in general.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




Similar articles
-
Enamel apatite crystallinity significantly contributes to mammalian dental adaptations.Sci Rep. 2018 Apr 3;8(1):5544. doi: 10.1038/s41598-018-23826-0. Sci Rep. 2018. PMID: 29615748 Free PMC article.
-
Enamel microarchitecture of a tribosphenic molar.J Morphol. 2010 Oct;271(10):1204-18. doi: 10.1002/jmor.10867. J Morphol. 2010. PMID: 20623522
-
To What Extent is Primate Second Molar Enamel Occlusal Morphology Shaped by the Enamel-Dentine Junction?PLoS One. 2015 Sep 25;10(9):e0138802. doi: 10.1371/journal.pone.0138802. eCollection 2015. PLoS One. 2015. PMID: 26406597 Free PMC article.
-
Cellular and chemical events during enamel maturation.Crit Rev Oral Biol Med. 1998;9(2):128-61. doi: 10.1177/10454411980090020101. Crit Rev Oral Biol Med. 1998. PMID: 9603233 Review.
-
Modern human molar enamel thickness and enamel-dentine junction shape.Arch Oral Biol. 2006 Nov;51(11):974-95. doi: 10.1016/j.archoralbio.2006.04.012. Epub 2006 Jun 30. Arch Oral Biol. 2006. PMID: 16814245 Review.
Cited by
-
Beyond the Map: Enamel Distribution Characterized from 3D Dental Topography.Front Physiol. 2017 Jul 21;8:524. doi: 10.3389/fphys.2017.00524. eCollection 2017. Front Physiol. 2017. PMID: 28785226 Free PMC article.
-
Crystallographic and Physicochemical Analysis of Bovine and Human Teeth Using X-ray Diffraction and Solid-State Nuclear Magnetic Resonance.J Funct Biomater. 2022 Nov 19;13(4):254. doi: 10.3390/jfb13040254. J Funct Biomater. 2022. PMID: 36412897 Free PMC article.
-
Enamel apatite crystallinity significantly contributes to mammalian dental adaptations.Sci Rep. 2018 Apr 3;8(1):5544. doi: 10.1038/s41598-018-23826-0. Sci Rep. 2018. PMID: 29615748 Free PMC article.
-
Rapid post-eruptive maturation of porcine enamel.Front Physiol. 2023 Feb 16;14:1099645. doi: 10.3389/fphys.2023.1099645. eCollection 2023. Front Physiol. 2023. PMID: 36875029 Free PMC article.
-
Proteomic Analyses Discern the Developmental Inclusion of Albumin in Pig Enamel: A New Model for Human Enamel Hypomineralization.Int J Mol Sci. 2023 Oct 25;24(21):15577. doi: 10.3390/ijms242115577. Int J Mol Sci. 2023. PMID: 37958567 Free PMC article.
References
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

