Nearly Monodisperse Insulator Cs4PbX6 (X = Cl, Br, I) Nanocrystals, Their Mixed Halide Compositions, and Their Transformation into CsPbX3 Nanocrystals
- PMID: 28196323
- PMCID: PMC5345893
- DOI: 10.1021/acs.nanolett.6b05262
Nearly Monodisperse Insulator Cs4PbX6 (X = Cl, Br, I) Nanocrystals, Their Mixed Halide Compositions, and Their Transformation into CsPbX3 Nanocrystals
Abstract
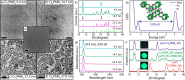
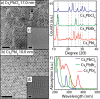
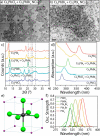
We have developed a colloidal synthesis of nearly monodisperse nanocrystals of pure Cs4PbX6 (X = Cl, Br, I) and their mixed halide compositions with sizes ranging from 9 to 37 nm. The optical absorption spectra of these nanocrystals display a sharp, high energy peak due to transitions between states localized in individual PbX64- octahedra. These spectral features are insensitive to the size of the particles and in agreement with the features of the corresponding bulk materials. Samples with mixed halide composition exhibit absorption bands that are intermediate in spectral position between those of the pure halide compounds. Furthermore, the absorption bands of intermediate compositions broaden due to the different possible combinations of halide coordination around the Pb2+ ions. Both observations are supportive of the fact that the [PbX6]4- octahedra are electronically decoupled in these systems. Because of the large band gap of Cs4PbX6 (>3.2 eV), no excitonic emission in the visible range was observed. The Cs4PbBr6 nanocrystals can be converted into green fluorescent CsPbBr3 nanocrystals by their reaction with an excess of PbBr2 with preservation of size and size distributions. The insertion of PbX2 into Cs4PbX6 provides a means of accessing CsPbX3 nanocrystals in a wide variety of sizes, shapes, and compositions, an important aspect for the development of precisely tuned perovskite nanocrystal inks.
Keywords: Cs4PbX6; halide perovskites; nanocrystals; synthesis; transformations.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
-
- Saliba M.; Orlandi S.; Matsui T.; Aghazada S.; Cavazzini M.; Correa-Baena J.-P.; Gao P.; Scopelliti R.; Mosconi E.; Dahmen K.-H.; De Angelis F.; Abate A.; Hagfeldt A.; Pozzi G.; Graetzel M.; Nazeeruddin M. K. Nat. Energy 2016, 1, 15017. 10.1038/nenergy.2015.17. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

