Therapeutic Hypothermia Provides Variable Protection against Behavioral Deficits after Neonatal Hypoxia-Ischemia: A Potential Role for Brain-Derived Neurotrophic Factor
- PMID: 28196356
- PMCID: PMC5607451
- DOI: 10.1159/000454949
Therapeutic Hypothermia Provides Variable Protection against Behavioral Deficits after Neonatal Hypoxia-Ischemia: A Potential Role for Brain-Derived Neurotrophic Factor
Abstract
Background: Despite treatment with therapeutic hypothermia (TH), infants who survive hypoxic ischemic (HI) encephalopathy (HIE) have persistent neurological abnormalities at school age. Protection by TH against HI brain injury is variable in both humans and animal models. Our current preclinical model of hypoxia-ischemia (HI) and TH displays this variability of outcomes in neuropathological and neuroimaging end points with some sexual dimorphism. The detailed behavioral phenotype of this model is unknown. Whether there is sexual dimorphism in certain behavioral domains is also not known. Brain-derived neurotrophic factor (BDNF) supports neuronal cell survival and repair but may also be a marker of injury. Here, we characterize the behavioral deficits after HI and TH stratified by sex, as well as late changes in BDNF and its correlation with memory impairment.
Methods: HI was induced in C57BL6 mice on postnatal day 10 (p10) (modified Vannucci model). Mice were randomized to TH (31°C) or normothermia (NT, 36°C) for 4 h after HI. Controls were anesthesia-exposed, age- and sex-matched littermates. Between p16 and p39, growth was followed, and behavioral testing was performed including reflexes (air righting, forelimb grasp and negative geotaxis) and sensorimotor, learning, and memory skills (open field, balance beam, adhesive removal, Y-maze tests, and object location task [OLT]). Correlations between mature BDNF levels in the forebrain and p42 memory outcomes were studied.
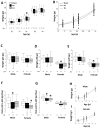
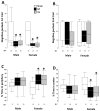
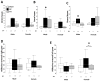
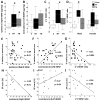
Results: Both male and female HI mice had an approximately 8-12% lower growth rate (g/day) than shams (p ≤ 0.01) by p39. TH ameliorated this growth failure in females but not in males. In female mice, HI injury prolonged the time spent at the periphery (open field) at p36 (p = 0.004), regardless of treatment. TH prevented motor impairments in the balance beam and adhesive removal tests in male and female mice, respectively (p ≤ 0.05). Male and female HI mice visited the new arm of the Y-maze 12.5% (p = 0.05) and 10% (p = 0.03) less often than shams, respectively. Male HI mice also had 35% lower exploratory preference score than sham (p ≤ 0.001) in the OLT. TH did not prevent memory impairments found with Y-maze testing or OLT in either sex (p ≤ 0.01) at p26. At p42, BDNF levels in the forebrain ipsilateral to the HI insult were 1.7- to 2-fold higher than BDNF levels in the sham forebrain, and TH did not prevent this increase. Higher BDNF levels in the forebrain ipsilateral to the insult correlated with worse performance in the Y-maze in both sexes and in OLT in male mice (p = 0.01).
Conclusions: TH provides benefit in specific domains of behavior following neonatal HI. In general, these benefits accrued to both males and females, but not in all areas. In some domains, such as memory, no benefit of TH was found. Late differences in individual BDNF levels may explain some of these findings.
Keywords: Astrocytes; Growth rate; Memory deficits; Primitive reflexes; Sensory-motor deficits.
© 2017 S. Karger AG, Basel.
Figures



Similar articles
-
Calbindin-1 Expression in the Hippocampus following Neonatal Hypoxia-Ischemia and Therapeutic Hypothermia and Deficits in Spatial Memory.Dev Neurosci. 2019 Mar 12:1-15. doi: 10.1159/000497056. Online ahead of print. Dev Neurosci. 2019. PMID: 30861522 Free PMC article.
-
Neonatal hypoxia-ischemia alters the events governing the hippocampal critical period of postnatal synaptic plasticity leading to deficits in working memory in mice.Neurobiol Dis. 2024 Nov;202:106722. doi: 10.1016/j.nbd.2024.106722. Epub 2024 Oct 30. Neurobiol Dis. 2024. PMID: 39486775 Free PMC article.
-
Long-term effects of enriched environment following neonatal hypoxia-ischemia on behavior, BDNF and synaptophysin levels in rat hippocampus: Effect of combined treatment with G-CSF.Brain Res. 2017 Jul 15;1667:55-67. doi: 10.1016/j.brainres.2017.05.004. Epub 2017 May 8. Brain Res. 2017. PMID: 28495306
-
Hydrogen-rich saline promotes microglia M2 polarization and complement-mediated synapse loss to restore behavioral deficits following hypoxia-ischemic in neonatal mice via AMPK activation.J Neuroinflammation. 2019 May 18;16(1):104. doi: 10.1186/s12974-019-1488-2. J Neuroinflammation. 2019. PMID: 31103039 Free PMC article. Review.
-
Sex-dependent consequences of neonatal brain hypoxia-ischemia in the rat.J Neurosci Res. 2017 Jan 2;95(1-2):409-421. doi: 10.1002/jnr.23828. J Neurosci Res. 2017. PMID: 27870406 Review.
Cited by
-
Early Neuroprotective Effects of Bovine Lactoferrin Associated with Hypothermia after Neonatal Brain Hypoxia-Ischemia in Rats.Int J Mol Sci. 2023 Oct 25;24(21):15583. doi: 10.3390/ijms242115583. Int J Mol Sci. 2023. PMID: 37958562 Free PMC article.
-
SMYD5 is a regulator of the mild hypothermia response.Cell Rep. 2024 Aug 27;43(8):114554. doi: 10.1016/j.celrep.2024.114554. Epub 2024 Jul 30. Cell Rep. 2024. PMID: 39083378 Free PMC article.
-
Ongoing loss of viable neurons for weeks after mild hypoxia-ischaemia.Brain Commun. 2025 Apr 18;7(2):fcaf153. doi: 10.1093/braincomms/fcaf153. eCollection 2025. Brain Commun. 2025. PMID: 40297712 Free PMC article.
-
Delayed injury of hippocampal interneurons after neonatal hypoxia-ischemia and therapeutic hypothermia in a murine model.Hippocampus. 2018 Aug;28(8):617-630. doi: 10.1002/hipo.22965. Hippocampus. 2018. PMID: 29781223 Free PMC article.
-
SMYD5 is a regulator of the mild hypothermia response.bioRxiv [Preprint]. 2024 Jul 12:2023.05.11.540170. doi: 10.1101/2023.05.11.540170. bioRxiv. 2024. Update in: Cell Rep. 2024 Aug 27;43(8):114554. doi: 10.1016/j.celrep.2024.114554. PMID: 37333301 Free PMC article. Updated. Preprint.
References
-
- Shankaran S. Outcomes of hypoxic-ischemic encephalopathy in neonates treated with hypothermia. Clin Perinatol. 2014;41:149–159. - PubMed
-
- Natarajan G, Shankaran S, Laptook AR, Pappas A, Bann CM, McDonald SA, Das A, Higgins RD, Hintz SR, Vohr BR Extended Hypothermia Subcommittee of the Eunice Kennedy Shriver National Institute of Child H, Human Development Neonatal Research N. Apgar scores at 10 min and outcomes at 6–7 years following hypoxic-ischaemic encephalopathy. Arch Dis Child Fetal Neonatal Ed. 2013;98:F473–479. - PMC - PubMed
-
- Shankaran S, Pappas A, McDonald SA, Vohr BR, Hintz SR, Yolton K, Gustafson KE, Leach TM, Green C, Bara R, Petrie Huitema CM, Ehrenkranz RA, Tyson JE, Das A, Hammond J, Peralta-Carcelen M, Evans PW, Heyne RJ, Wilson-Costello DE, Vaucher YE, Bauer CR, Dusick AM, Adams-Chapman I, Goldstein RF, Guillet R, Papile LA, Higgins RD Eunice Kennedy Shriver NNRN. Childhood outcomes after hypothermia for neonatal encephalopathy. N Engl J Med. 2012;366:2085–2092. - PMC - PubMed
-
- Edwards AD, Brocklehurst P, Gunn AJ, Halliday H, Juszczak E, Levene M, Strohm B, Thoresen M, Whitelaw A, Azzopardi D. Neurological outcomes at 18 months of age after moderate hypothermia for perinatal hypoxic ischaemic encephalopathy: synthesis and meta-analysis of trial data. BMJ. 2010;340:c363. - PMC - PubMed
-
- Burton VJ, Gerner G, Cristofalo E, Chung SE, Jennings JM, Parkinson C, Koehler RC, Chavez-Valdez R, Johnston MV, Northington FJ, Lee JK. A pilot cohort study of cerebral autoregulation and 2-year neurodevelopmental outcomes in neonates with hypoxic-ischemic encephalopathy who received therapeutic hypothermia. BMC Neurol. 2015;15:209. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

