Metabolite toxicity determines the pace of molecular evolution within microbial populations
- PMID: 28196465
- PMCID: PMC5310025
- DOI: 10.1186/s12862-017-0906-2
Metabolite toxicity determines the pace of molecular evolution within microbial populations
Abstract
Background: The production of toxic metabolites has shaped the spatial and temporal arrangement of metabolic processes within microbial cells. While diverse solutions to mitigate metabolite toxicity have evolved, less is known about how evolution itself is affected by metabolite toxicity. We hypothesized that the pace of molecular evolution should increase as metabolite toxicity increases. At least two mechanisms could cause this. First, metabolite toxicity could increase the mutation rate. Second, metabolite toxicity could increase the number of available mutations with large beneficial effects that selection could act upon (e.g., mutations that provide tolerance to toxicity), which consequently would increase the rate at which those mutations increase in frequency.
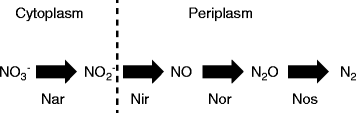
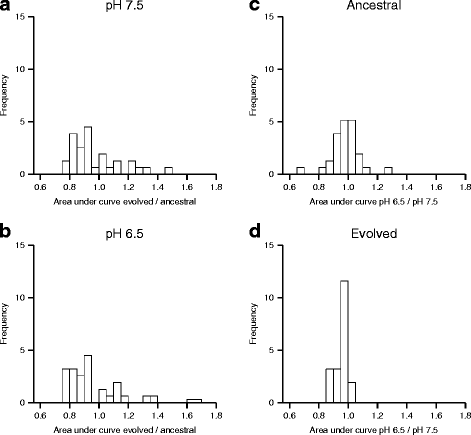
Results: We tested this hypothesis by experimentally evolving the bacterium Pseudomonas stutzeri under denitrifying conditions. The metabolite nitrite accumulates during denitrification and has pH-dependent toxic effects, which allowed us to evolve P. stutzeri at different magnitudes of nitrite toxicity. We demonstrate that increased nitrite toxicity results in an increased pace of molecular evolution. We further demonstrate that this increase is generally due to an increased number of available mutations with large beneficial effects and not to an increased mutation rate.
Conclusions: Our results demonstrate that the production of toxic metabolites can have important impacts on the evolutionary processes of microbial cells. Given the ubiquity of toxic metabolites, they could also have implications for understanding the evolutionary histories of biological organisms.
Keywords: Denitrification; Experimental evolution; Microbial populations; Molecular evolution; Nitrite toxicity.
Figures





References
-
- de Lorenzo V, Sekowska A, Danchin A. Chemical reactivity drives spatiotemporal organisation of bacterial metabolism. FEMS Microbiol Rev. 2015;39:96–119. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

