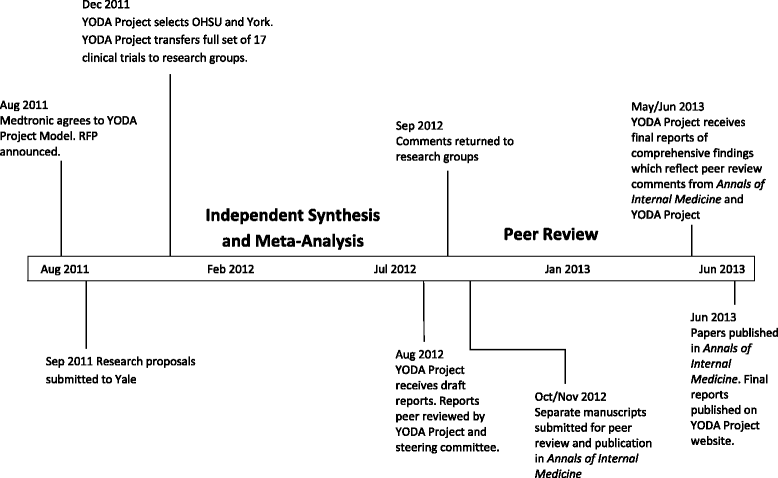
Comparison of two independent systematic reviews of trials of recombinant human bone morphogenetic protein-2 (rhBMP-2): the Yale Open Data Access Medtronic Project
- PMID: 28196521
- PMCID: PMC5310069
- DOI: 10.1186/s13643-017-0422-x
Comparison of two independent systematic reviews of trials of recombinant human bone morphogenetic protein-2 (rhBMP-2): the Yale Open Data Access Medtronic Project
Abstract
Background: It is uncertain whether the replication of systematic reviews, particularly those with the same objectives and resources, would employ similar methods and/or arrive at identical findings. We compared the results and conclusions of two concurrent systematic reviews undertaken by two independent research teams provided with the same objectives, resources, and individual participant-level data.
Methods: Two centers in the USA and UK were each provided with participant-level data on 17 multi-site clinical trials of recombinant human bone morphogenetic protein-2 (rhBMP-2). The teams were blinded to each other's methods and findings until after publication. We conducted a retrospective structured comparison of the results of the two systematic reviews. The main outcome measures included (1) trial inclusion criteria; (2) statistical methods; (3) summary efficacy and risk estimates; and (4) conclusions.
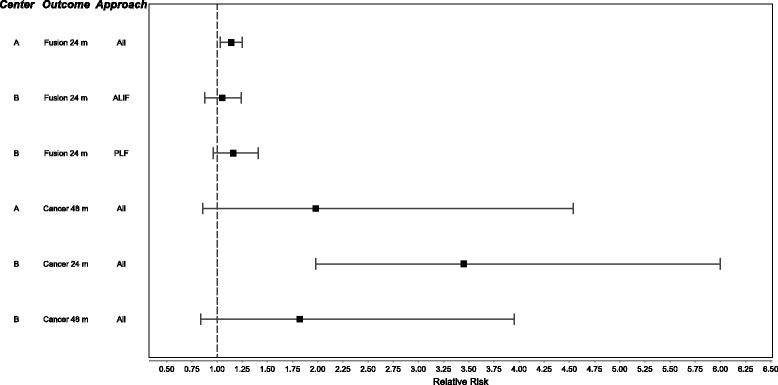
Results: The two research teams' meta-analyses inclusion criteria were broadly similar but differed slightly in trial inclusion and research methodology. They obtained similar results in summary estimates of most clinical outcomes and adverse events. Center A incorporated all trials into summary estimates of efficacy and harms, while Center B concentrated on analyses stratified by surgical approach. Center A found a statistically significant, but small, benefit whereas Center B reported no advantage. In the analysis of harms, neither showed an increased cancer risk at 48 months, although Center B reported a significant increase at 24 months. Conclusions reflected these differences in summary estimates of benefit balanced with small but potentially important risk of harm.
Conclusions: Two independent groups given the same research objectives, data, resources, funding, and time produced broad general agreement but differed in several areas. These differences, the importance of which is debatable, indicate the value of the availability of data to allow for more than a single approach and a single interpretation of the data.
Systematic review registration: PROSPERO CRD42012002040 and CRD42012001907 .
Keywords: Data interpretation; Data sharing; Meta-analysis; Systematic review.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources