Comparative genomics reveals high biological diversity and specific adaptations in the industrially and medically important fungal genus Aspergillus
- PMID: 28196534
- PMCID: PMC5307856
- DOI: 10.1186/s13059-017-1151-0
Comparative genomics reveals high biological diversity and specific adaptations in the industrially and medically important fungal genus Aspergillus
Abstract
Background: The fungal genus Aspergillus is of critical importance to humankind. Species include those with industrial applications, important pathogens of humans, animals and crops, a source of potent carcinogenic contaminants of food, and an important genetic model. The genome sequences of eight aspergilli have already been explored to investigate aspects of fungal biology, raising questions about evolution and specialization within this genus.
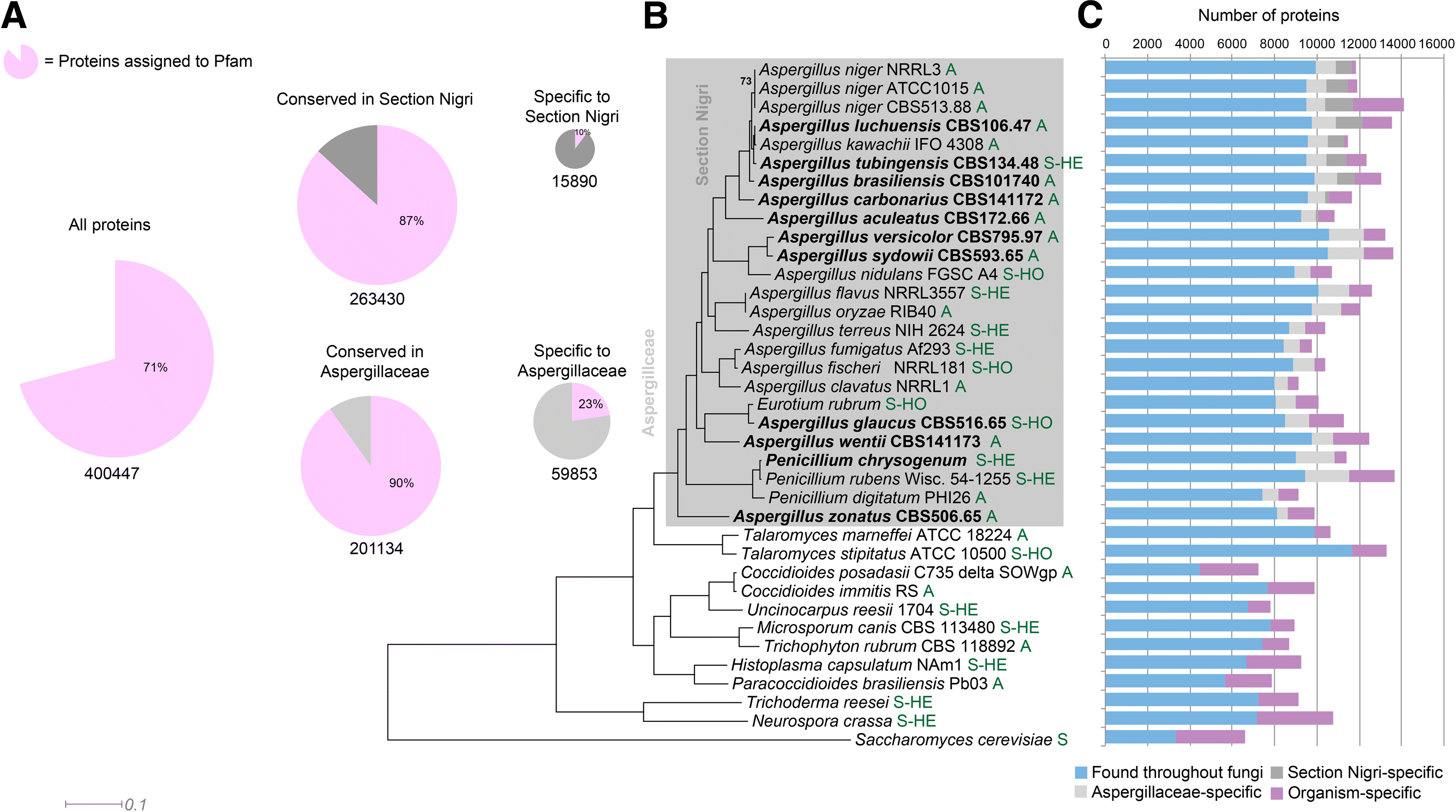
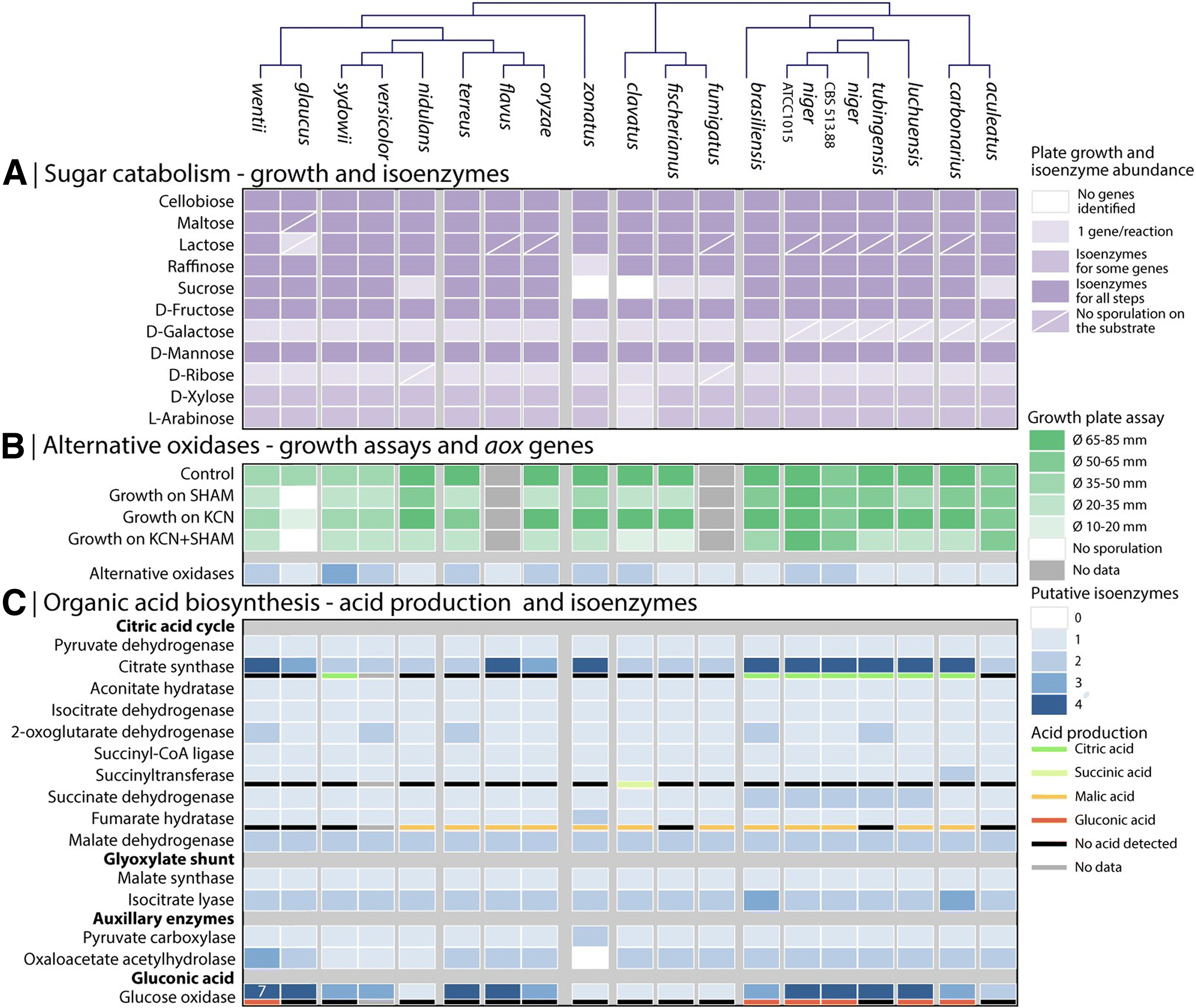
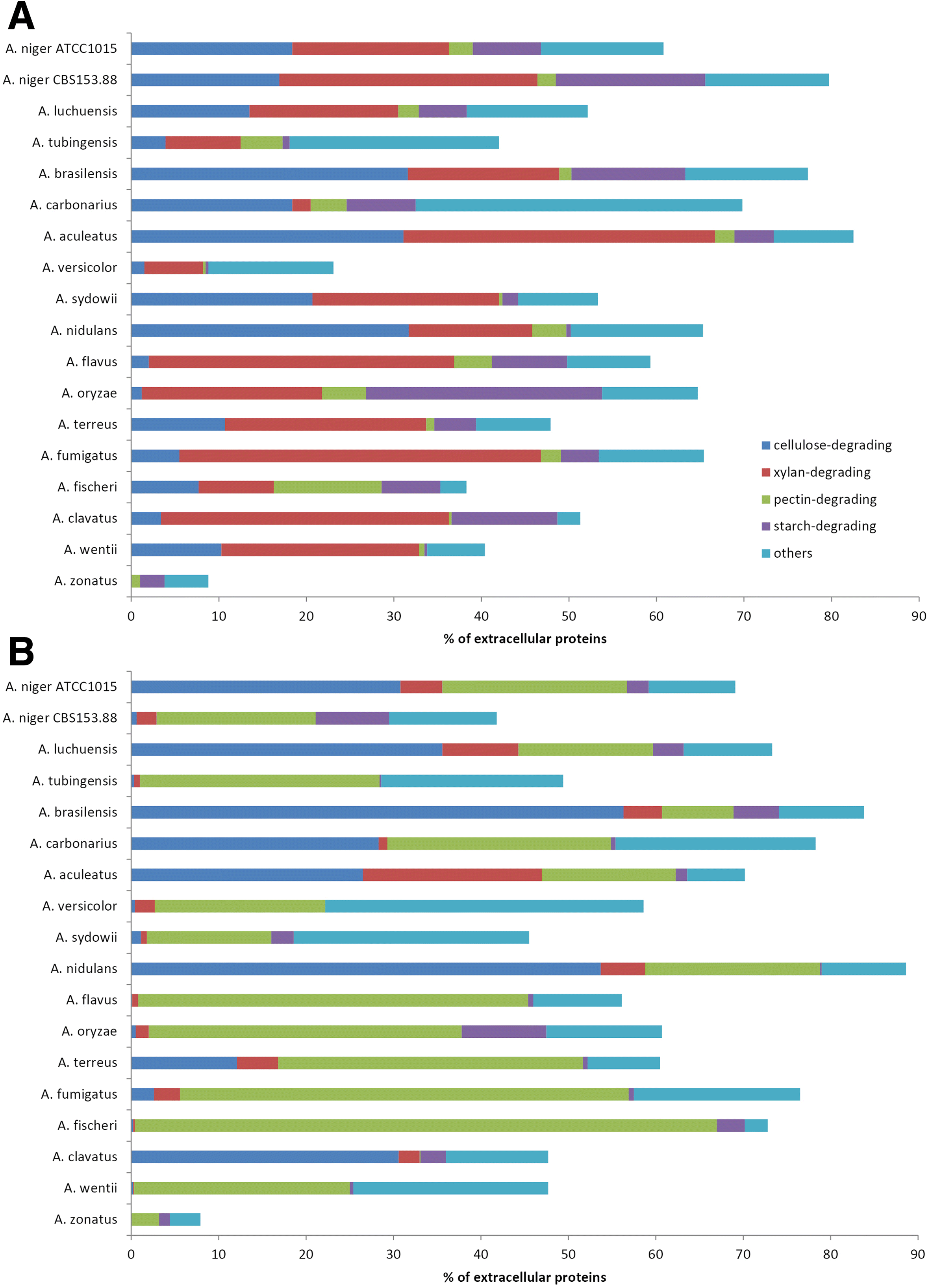
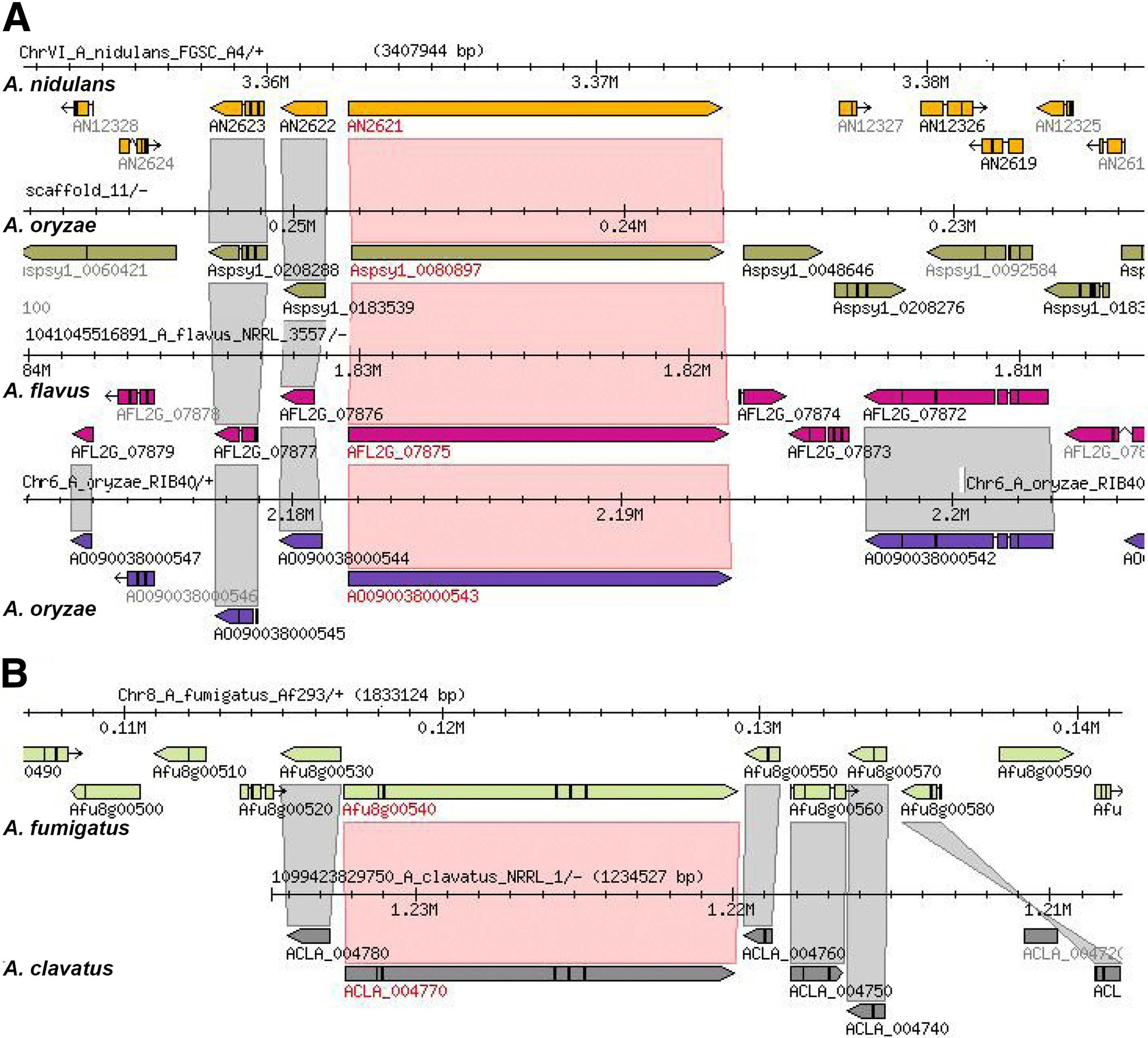
Results: We have generated genome sequences for ten novel, highly diverse Aspergillus species and compared these in detail to sister and more distant genera. Comparative studies of key aspects of fungal biology, including primary and secondary metabolism, stress response, biomass degradation, and signal transduction, revealed both conservation and diversity among the species. Observed genomic differences were validated with experimental studies. This revealed several highlights, such as the potential for sex in asexual species, organic acid production genes being a key feature of black aspergilli, alternative approaches for degrading plant biomass, and indications for the genetic basis of stress response. A genome-wide phylogenetic analysis demonstrated in detail the relationship of the newly genome sequenced species with other aspergilli.
Conclusions: Many aspects of biological differences between fungal species cannot be explained by current knowledge obtained from genome sequences. The comparative genomics and experimental study, presented here, allows for the first time a genus-wide view of the biological diversity of the aspergilli and in many, but not all, cases linked genome differences to phenotype. Insights gained could be exploited for biotechnological and medical applications of fungi.
Keywords: Aspergillus; Comparative genomics; Fungal biology; Genome sequencing.
Figures


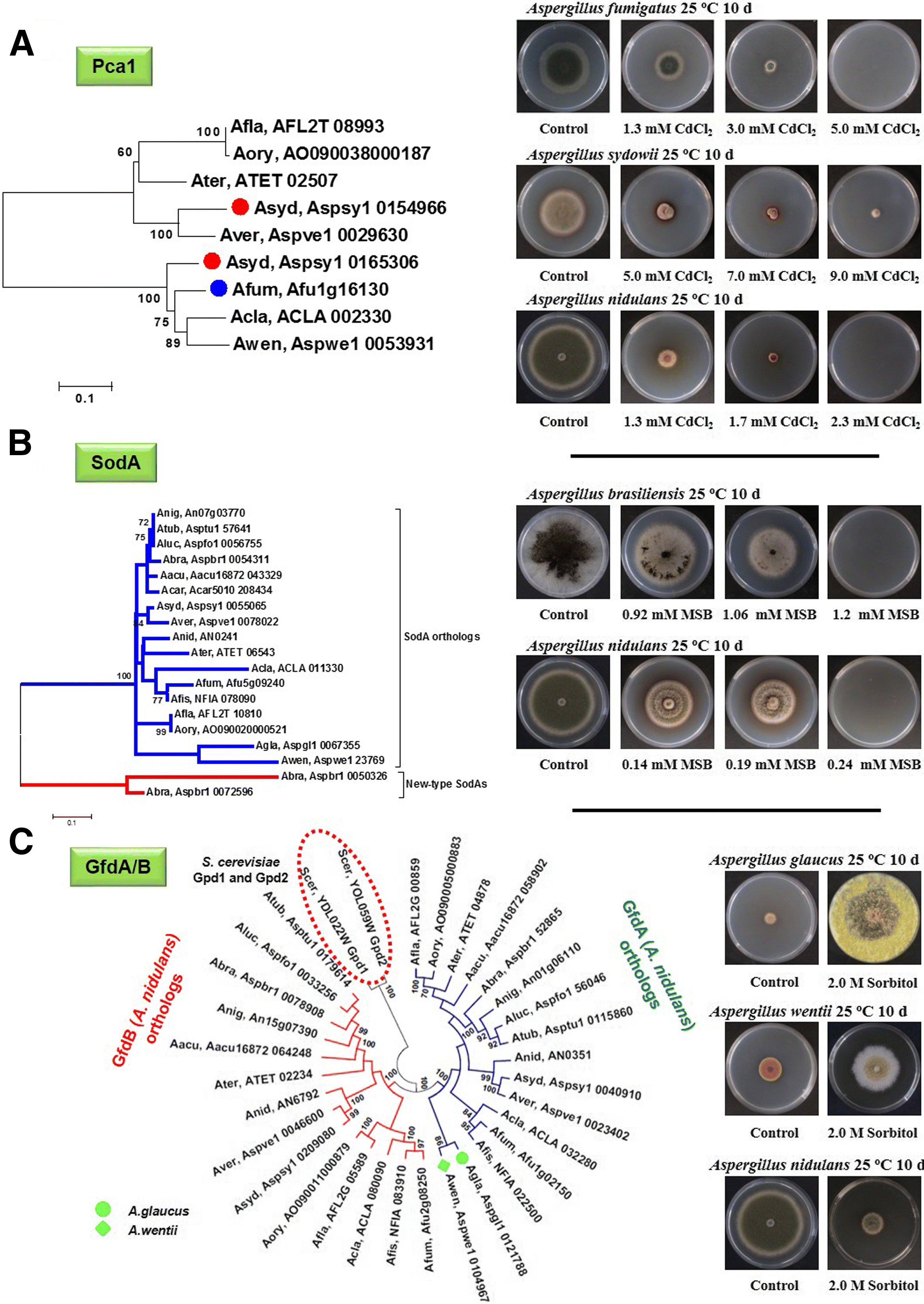
 ) and A. fumigatus (
) and A. fumigatus ( ) Pca1 orthologs (a) and putative A. glaucus (
) Pca1 orthologs (a) and putative A. glaucus ( ) and A. wentii (
) and A. wentii ( ) GfdA orthologs (c) are indicated by symbols presented in the parentheses. Hypothetical new-type SodA enzymes found in A. brasiliensis are indicated by red lines in (b). Pca1, SodA, and GfdA/B orthologs are indicated by four-letter species name identifiers (listed in Additional file 15) and locus IDs as found in AspGD (
) GfdA orthologs (c) are indicated by symbols presented in the parentheses. Hypothetical new-type SodA enzymes found in A. brasiliensis are indicated by red lines in (b). Pca1, SodA, and GfdA/B orthologs are indicated by four-letter species name identifiers (listed in Additional file 15) and locus IDs as found in AspGD (
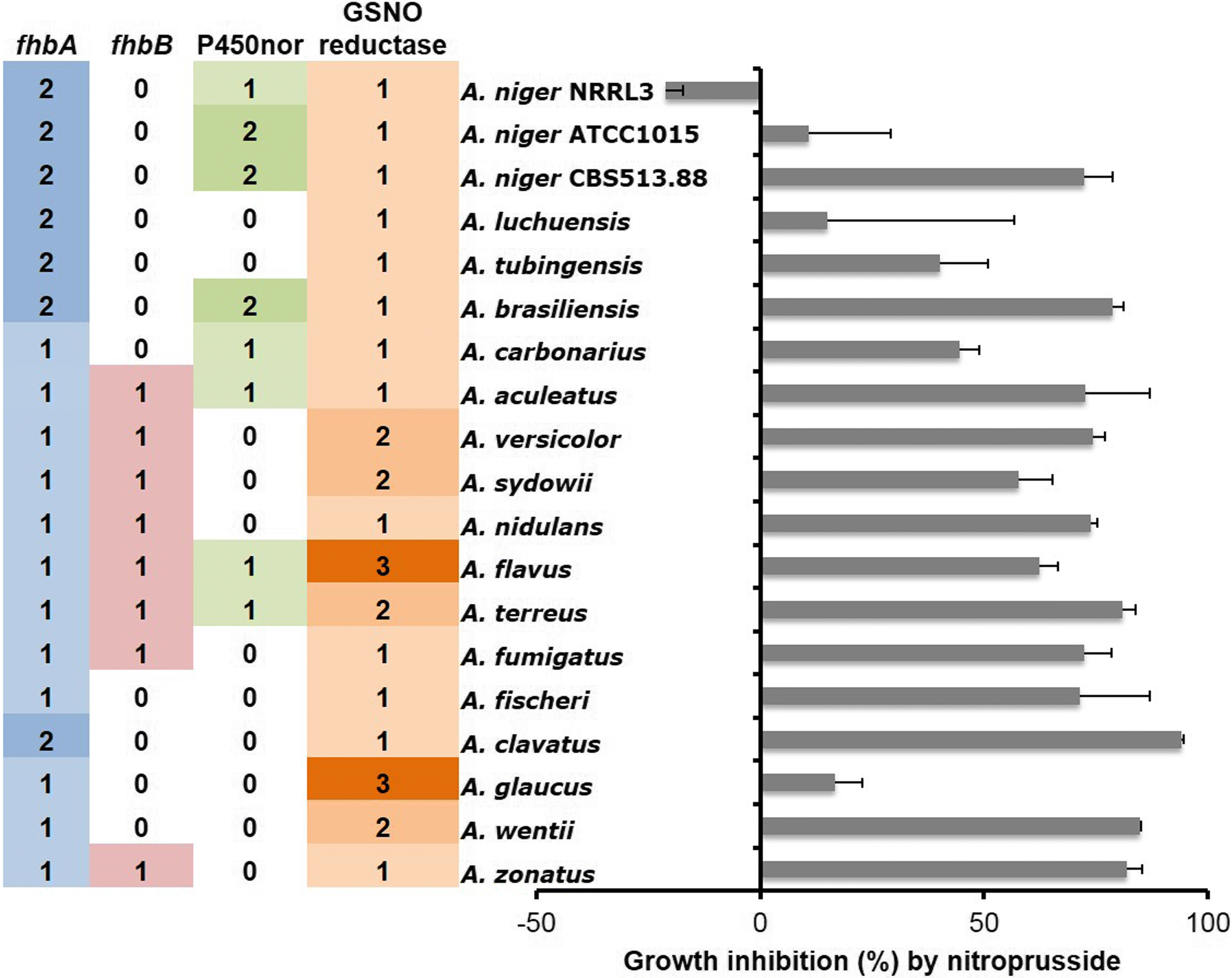
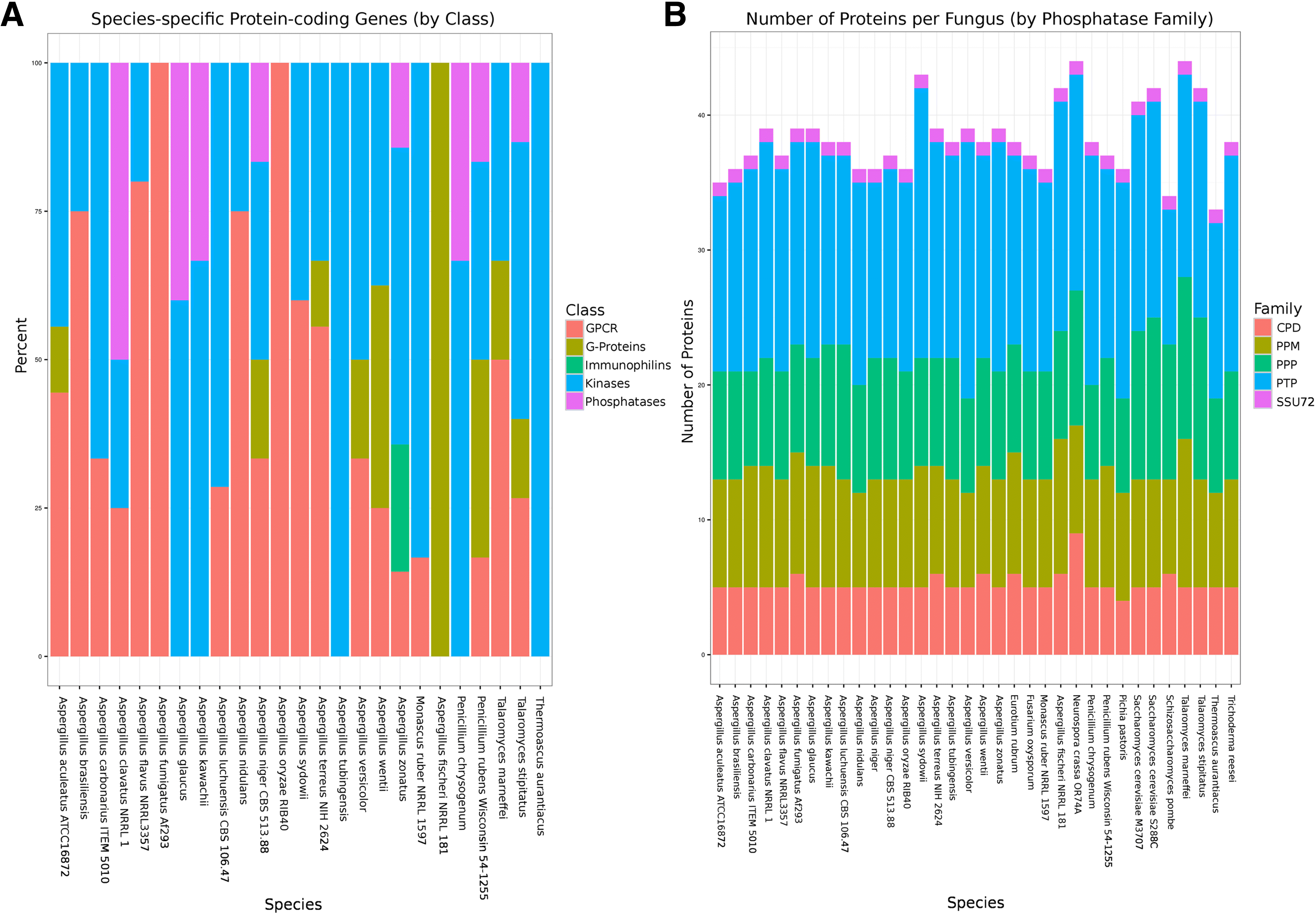
References
-
- Scazzocchio C. Aspergillus, a multifacted genus In: Schaechter M, editor. Encyclopaedia of Microbiology. Amsterdam: Elsevier; 2009. p. 401-420.
-
- Geiser DM, Samson RA, Varga J, Rokas A, Witiak SM. A review of molecular phylogenetics in Aspergillus, and prospects for a robust genus-wide phylogeny. In: Varga J, Samson RA, editors. Aspergillus in the genomic era. Wageningen: Wageningen Academic Publishers; 2008. pp. 17–32.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

