GATA6 Plays an Important Role in the Induction of Human Definitive Endoderm, Development of the Pancreas, and Functionality of Pancreatic β Cells
- PMID: 28196690
- PMCID: PMC5355564
- DOI: 10.1016/j.stemcr.2016.12.026
GATA6 Plays an Important Role in the Induction of Human Definitive Endoderm, Development of the Pancreas, and Functionality of Pancreatic β Cells
Abstract
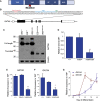
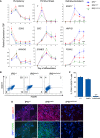
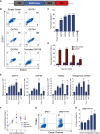
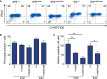
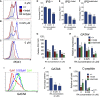
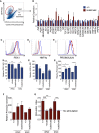
Induced pluripotent stem cells were created from a pancreas agenesis patient with a mutation in GATA6. Using genome-editing technology, additional stem cell lines with mutations in both GATA6 alleles were generated and demonstrated a severe block in definitive endoderm induction, which could be rescued by re-expression of several different GATA family members. Using the endodermal progenitor stem cell culture system to bypass the developmental block at the endoderm stage, cell lines with mutations in one or both GATA6 alleles could be differentiated into β-like cells but with reduced efficiency. Use of suboptimal doses of retinoic acid during pancreas specification revealed a more severe phenotype, more closely mimicking the patient's disease. GATA6 mutant β-like cells fail to secrete insulin upon glucose stimulation and demonstrate defective insulin processing. These data show that GATA6 plays a critical role in endoderm and pancreas specification and β-like cell functionality in humans.
Keywords: GATA6; definitive endoderm; pancreatic agenesis; retinoic acid; stem cells; β cell.
Copyright © 2017 The Authors. Published by Elsevier Inc. All rights reserved.
Figures

Comment in
-
Gotta Have GATA for Human Pancreas Development.Cell Stem Cell. 2017 May 4;20(5):577-579. doi: 10.1016/j.stem.2017.04.004. Cell Stem Cell. 2017. PMID: 28475878
Similar articles
-
Gotta Have GATA for Human Pancreas Development.Cell Stem Cell. 2017 May 4;20(5):577-579. doi: 10.1016/j.stem.2017.04.004. Cell Stem Cell. 2017. PMID: 28475878
-
GATA6 Cooperates with EOMES/SMAD2/3 to Deploy the Gene Regulatory Network Governing Human Definitive Endoderm and Pancreas Formation.Stem Cell Reports. 2019 Jan 8;12(1):57-70. doi: 10.1016/j.stemcr.2018.12.003. Stem Cell Reports. 2019. PMID: 30629940 Free PMC article.
-
Genome Editing in hPSCs Reveals GATA6 Haploinsufficiency and a Genetic Interaction with GATA4 in Human Pancreatic Development.Cell Stem Cell. 2017 May 4;20(5):675-688.e6. doi: 10.1016/j.stem.2017.01.001. Epub 2017 Feb 9. Cell Stem Cell. 2017. PMID: 28196600 Free PMC article.
-
Conversion of embryonic stem cells into pancreatic beta-cell surrogates guided by ontogeny.Regen Med. 2006 May;1(3):327-36. doi: 10.2217/17460751.1.3.327. Regen Med. 2006. PMID: 17465786 Review.
-
In vitro models of pancreatic differentiation using embryonic stem or induced pluripotent stem cells.Congenit Anom (Kyoto). 2011 Mar;51(1):21-5. doi: 10.1111/j.1741-4520.2010.00307.x. Congenit Anom (Kyoto). 2011. PMID: 21129040 Review.
Cited by
-
Genome editing of human pancreatic beta cell models: problems, possibilities and outlook.Diabetologia. 2019 Aug;62(8):1329-1336. doi: 10.1007/s00125-019-4908-z. Epub 2019 Jun 3. Diabetologia. 2019. PMID: 31161346 Free PMC article. Review.
-
GATA6 is essential for endoderm formation from human pluripotent stem cells.Biol Open. 2017 Jul 15;6(7):1084-1095. doi: 10.1242/bio.026120. Biol Open. 2017. PMID: 28606935 Free PMC article.
-
CRISPR screening uncovers a central requirement for HHEX in pancreatic lineage commitment and plasticity restriction.Nat Cell Biol. 2022 Jul;24(7):1064-1076. doi: 10.1038/s41556-022-00946-4. Epub 2022 Jul 4. Nat Cell Biol. 2022. PMID: 35787684 Free PMC article.
-
Leveraging the strengths of mice, human stem cells, and organoids to model pancreas development and diabetes.Front Endocrinol (Lausanne). 2022 Oct 21;13:1042611. doi: 10.3389/fendo.2022.1042611. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 36339450 Free PMC article. Review.
-
GATA6-AS1 Regulates GATA6 Expression to Modulate Human Endoderm Differentiation.Stem Cell Reports. 2020 Sep 8;15(3):694-705. doi: 10.1016/j.stemcr.2020.07.014. Epub 2020 Aug 13. Stem Cell Reports. 2020. PMID: 32795420 Free PMC article.
References
-
- Bonnefond A., Sand O., Guerin B., Durand E., De Graeve F., Huyvaert M., Rachdi L., Kerr-Conte J., Pattou F., Vaxillaire M. GATA6 inactivating mutations are associated with heart defects and, inconsistently, with pancreatic agenesis and diabetes. Diabetologia. 2012;55:2845–2847. - PubMed
-
- Brewer A., Gove C., Davies A., McNulty C., Barrow D., Koutsourakis M., Farzaneh F., Pizzey J., Bomford A., Patient R. The human and mouse GATA-6 genes utilize two promoters and two initiation codons. J. Biol. Chem. 1999;274:38004–38016. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

