Cerebrospinal fluid markers detect Alzheimer's disease in nonamnestic dementia
- PMID: 28196768
- PMCID: PMC5787859
- DOI: 10.1016/j.jalz.2017.01.006
Cerebrospinal fluid markers detect Alzheimer's disease in nonamnestic dementia
Abstract
Introduction: The accuracy of cerebrospinal fluid (CSF) biomarkers for detecting Alzheimer's disease (AD) pathology has not been fully validated in autopsied nonamnestic dementias.
Methods: We retrospectively evaluated CSF amyloid β 1-42, phosphorylated-tau, and amyloid-tau index as predictors of Alzheimer pathology in patients with primary progressive aphasia, frontotemporal dementia, and progressive supranuclear palsy.
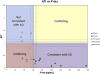
Results: Nineteen nonamnestic autopsied cases with relevant CSF values were included. At autopsy, nine had AD and 10 had non-AD pathologies. All six patients whose combined CSF phosphorylated-tau and amyloid β levels were "consistent with AD" had postmortem Alzheimer pathology. The two patients whose biomarker values were "not consistent with AD" had non-AD pathologies. The CSF values of the remaining eight non-AD cases were in conflicting or borderline ranges.
Discussion: CSF biomarkers reliably identified Alzheimer pathology in nonamnestic dementias and may be useful as a screening measure for inclusion of nonamnestic cases into Alzheimer's trials.
Keywords: Atypical Alzheimer's disease; Behavioral variant frontotemporal dementia; Neuropathology; Primary progressive aphasia; Progressive supranuclear palsy.
Copyright © 2017 the Alzheimer's Association. Published by Elsevier Inc. All rights reserved.
Figures
References
-
- Andreasen N, Minthon L, Davidsson P, Vanmechelen E, Vanderstichele H, Winblad B, et al. Evaluation of CSF-tau and CSF-Abeta42 as diagnostic markers for Alzheimer disease in clinical practice. Arch Neurol. 2001;58:373–9. - PubMed
-
- Mesulam MM. Primary progressive aphasia. Ann Neurol. 2001;49:425–32. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

