Metabolic remodeling during the loss and acquisition of pluripotency
- PMID: 28196802
- PMCID: PMC5312031
- DOI: 10.1242/dev.128389
Metabolic remodeling during the loss and acquisition of pluripotency
Abstract
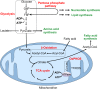
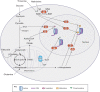
Pluripotent cells from the early stages of embryonic development have the unlimited capacity to self-renew and undergo differentiation into all of the cell types of the adult organism. These properties are regulated by tightly controlled networks of gene expression, which in turn are governed by the availability of transcription factors and their interaction with the underlying epigenetic landscape. Recent data suggest that, perhaps unexpectedly, some key epigenetic marks, and thereby gene expression, are regulated by the levels of specific metabolites. Hence, cellular metabolism plays a vital role beyond simply the production of energy, and may be involved in the regulation of cell fate. In this Review, we discuss the metabolic changes that occur during the transitions between different pluripotent states both in vitro and in vivo, including during reprogramming to pluripotency and the onset of differentiation, and we discuss the extent to which distinct metabolites might regulate these transitions.
Keywords: Epigenetics; Metabolic remodeling; Stem cell.
© 2017. Published by The Company of Biologists Ltd.
Conflict of interest statement
The authors declare no competing or financial interests.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

