Bone Marrow-Derived MicroRNA-223 Works as an Endocrine Genetic Signal in Vascular Endothelial Cells and Participates in Vascular Injury From Kawasaki Disease
- PMID: 28196816
- PMCID: PMC5523776
- DOI: 10.1161/JAHA.116.004878
Bone Marrow-Derived MicroRNA-223 Works as an Endocrine Genetic Signal in Vascular Endothelial Cells and Participates in Vascular Injury From Kawasaki Disease
Abstract
Background: Kawasaki disease (KD) is now the most common cause of acquired cardiac disease in children due to permanent coronary artery damage with unknown etiology. The study sought to determine the role of blood microRNA miR-223 in KD and KD-induced injuries in vascular endothelial cells (ECs) as well as the mechanisms involved.
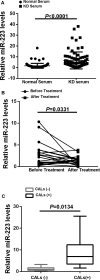
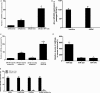
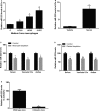
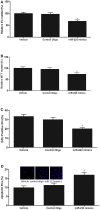
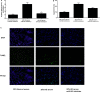
Methods and results: MicroRNA profiles in serum from patients with KD and from healthy controls were assessed by microarray analysis. We noted that multiple serum microRNAs were aberrantly expressed in KD, among them miR-223, which was the most upregulated abundant serum microRNA. We found that bone marrow-derived blood cells (leukocytes and platelets) were able to secrete miR-223 into serum. Vascular ECs had no endogenous miR-223; however, the blood cell-secreted serum miR-223 could enter into the vascular ECs in the vascular walls. The exogenous miR-223 had strong biological effects on EC functions via its target genes such as IGF1R. Interestingly, KD-induced EC injuries were related to increased miR-223 because they were inhibited by miR-223 knockdown. Finally, these observations were verified using miR-223 knockout mice and the chimeric mice generated by transplantation of bone marrow from miR-223 knockout mice into wild-type mice.
Conclusions: In KD patients, the levels of blood cell-derived miR-223 in ECs are significantly increased. The increased miR-223 in ECs could work as a novel endocrine genetic signal and participate in vascular injury of KD. MiR-223 may provide a novel mechanism and a new therapeutic target for vascular complication of KD.
Keywords: Kawasaki disease; blood cells; endothelial cells; miR‐223; microRNA; vascular inflammation.
© 2017 The Authors. Published on behalf of the American Heart Association, Inc., by Wiley Blackwell.
Figures

References
-
- Newburger JW, Takahashi M, Burns JC. Kawasaki disease. J Am Coll Cardiol. 2016;67:1738–1749. - PubMed
-
- Chen KY, Curtis N, Dahdah N, Kowalski R, Cheung M, Burgner DP, Kowalski R. Kawasaki disease and cardiovascular risk: a comprehensive review of subclinical vascular changes in the longer term. Acta Paediatr. 2016;105:752–761. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

