Conformational changes in the activation loop of mitochondrial glutaminase C: A direct fluorescence readout that distinguishes the binding of allosteric inhibitors from activators
- PMID: 28196863
- PMCID: PMC5391743
- DOI: 10.1074/jbc.M116.758219
Conformational changes in the activation loop of mitochondrial glutaminase C: A direct fluorescence readout that distinguishes the binding of allosteric inhibitors from activators
Abstract
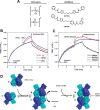
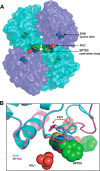
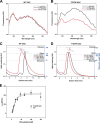
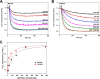
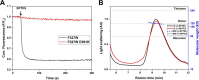
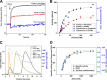
The first step in glutamine catabolism is catalysis by the mitochondrial enzyme glutaminase, with a specific isoform, glutaminase C (GAC), being highly expressed in cancer cells. GAC activation requires the formation of homotetramers, promoted by anionic allosteric activators such as inorganic phosphate. This leads to the proper orientation of a flexible loop proximal to the dimer-dimer interface that is essential for catalysis (i.e. the "activation loop"). A major class of allosteric inhibitors of GAC, with the prototype being bis-2-(5-phenylacetamido-1,2,4-thiadiazol-2-yl)ethyl sulfide (BPTES) and the related molecule CB-839, binds to the activation loop and induces the formation of an inactive tetramer (two inhibitors bound per active tetramer). Here we describe a direct readout for monitoring the dynamics of the activation loop of GAC in response to these allosteric inhibitors, as well as allosteric activators, through the substitution of phenylalanine at position 327 with tryptophan (F327W). The tryptophan fluorescence of the GAC(F327W) mutant undergoes a marked quenching upon the binding of BPTES or CB-839, yielding titration profiles that make it possible to measure the binding affinities of these inhibitors for the enzyme. Allosteric activators like phosphate induce the opposite effect (i.e. fluorescence enhancement). These results describe direct readouts for the binding of the BPTES class of allosteric inhibitors as well as for inorganic phosphate and related activators of GAC, which should facilitate screening for additional modulators of this important metabolic enzyme.
Keywords: fluorescence; glutaminase; glutamine; metabolism; tryptophan.
© 2017 by The American Society for Biochemistry and Molecular Biology, Inc.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures



References
-
- Krall A. S., and Christofk H. R. (2015) Rethinking glutamine addiction. Nat. Cell Biol. 17, 1515–1517 - PubMed
-
- DeBerardinis R. J., Lum J. J., Hatzivassiliou G., and Thompson C. B. (2008) The biology of cancer: metabolic reprogramming fuels cell growth and proliferation. Cell Metab. 7, 11–20 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

