SGLT2 Protein Expression Is Increased in Human Diabetic Nephropathy: SGLT2 PROTEIN INHIBITION DECREASES RENAL LIPID ACCUMULATION, INFLAMMATION, AND THE DEVELOPMENT OF NEPHROPATHY IN DIABETIC MICE
- PMID: 28196866
- PMCID: PMC5392679
- DOI: 10.1074/jbc.M117.779520
SGLT2 Protein Expression Is Increased in Human Diabetic Nephropathy: SGLT2 PROTEIN INHIBITION DECREASES RENAL LIPID ACCUMULATION, INFLAMMATION, AND THE DEVELOPMENT OF NEPHROPATHY IN DIABETIC MICE
Abstract
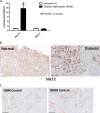
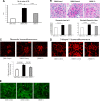
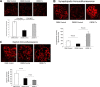
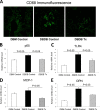
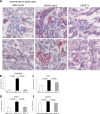
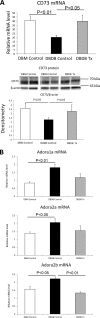
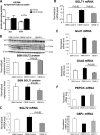
There is very limited human renal sodium gradient-dependent glucose transporter protein (SGLT2) mRNA and protein expression data reported in the literature. The first aim of this study was to determine SGLT2 mRNA and protein levels in human and animal models of diabetic nephropathy. We have found that the expression of SGLT2 mRNA and protein is increased in renal biopsies from human subjects with diabetic nephropathy. This is in contrast to db-db mice that had no changes in renal SGLT2 protein expression. Furthermore, the effect of SGLT2 inhibition on renal lipid content and inflammation is not known. The second aim of this study was to determine the potential mechanisms of beneficial effects of SGLT2 inhibition in the progression of diabetic renal disease. We treated db/db mice with a selective SGLT2 inhibitor JNJ 39933673. We found that SGLT2 inhibition caused marked decreases in systolic blood pressure, kidney weight/body weight ratio, urinary albumin, and urinary thiobarbituric acid-reacting substances. SGLT2 inhibition prevented renal lipid accumulation via inhibition of carbohydrate-responsive element-binding protein-β, pyruvate kinase L, SCD-1, and DGAT1, key transcriptional factors and enzymes that mediate fatty acid and triglyceride synthesis. SGLT2 inhibition also prevented inflammation via inhibition of CD68 macrophage accumulation and expression of p65, TLR4, MCP-1, and osteopontin. These effects were associated with reduced mesangial expansion, accumulation of the extracellular matrix proteins fibronectin and type IV collagen, and loss of podocyte markers WT1 and synaptopodin, as determined by immunofluorescence microscopy. In summary, our study showed that SGLT2 inhibition modulates renal lipid metabolism and inflammation and prevents the development of nephropathy in db/db mice.
Keywords: diabetes; diabetic nephropathy; glucose transport; immunochemistry; inflammation; lipid synthesis; microscopic imaging.
© 2017 by The American Society for Biochemistry and Molecular Biology, Inc.
Conflict of interest statement
This work was funded in part by a Medical School grant provided by Janssen Research & Development, LLC, Raritan, NJ (to M. L.)
Figures
References
-
- Odermatt A. (2011) The Western-style diet: a major risk factor for impaired kidney function and chronic kidney disease. Am. J. Physiol. Renal Physiol. 301, F919–F931 - PubMed
-
- Forbes J. M., Coughlan M. T., and Cooper M. E. (2008) Oxidative stress as a major culprit in kidney disease in diabetes. Diabetes 57, 1446–1454 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

