MUC16 Regulates TSPYL5 for Lung Cancer Cell Growth and Chemoresistance by Suppressing p53
- PMID: 28196872
- PMCID: PMC5511558
- DOI: 10.1158/1078-0432.CCR-16-2530
MUC16 Regulates TSPYL5 for Lung Cancer Cell Growth and Chemoresistance by Suppressing p53
Abstract
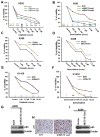
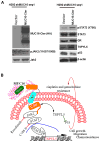
Purpose: MUC16, a tumor biomarker and cell surface-associated mucin, is overexpressed in various cancers; however, its role in lung cancer pathogenesis is unknown. Here, we have explored the mechanistic role of MUC16 in lung cancer.Experimental Design: To identify the functional role of MUC16, stable knockdown was carried in lung cancer cells with two different shRNAs. Clinical significance of MUC16 was evaluated in lung cancer patient tissues using IHC. We have generated genetically engineered mouse model (KrasG12D; AdCre) to evaluate the preclinical significance of MUC16.Results: MUC16 was overexpressed (P = 0.03) in lung cancer as compared with normal tissues. MUC16 knockdown (KD) in lung cancer cell lines decreased the in vitro growth rate (P < 0.05), migration (P < 0.001), and in vivo tumor growth (P = 0.007), whereas overexpression of MUC16-carboxyl terminal (MUC16-Cter) resulted in increased growth rate (P < 0.001). Transcriptome analysis of MUC16 KD showed a downregulation (P = 0.005) of TSPYL5 gene, which encodes for a testis-specific Y-like protein. Rescue studies via overexpression of MUC16-Cter in MUC16 KD cells showed activation of signaling proteins, such as JAK2 (Y1007/1008), STAT3 (Y705), and glucocorticoid receptor (GR), which constitutes an important axis for the regulation of TSPYL5 for oncogenic process. Further, inhibition of STAT3 (Y705) led to decreased GR and TSPYL5, suggesting that MUC16 regulates TSPYL5 through the JAK2/STAT3/GR axis. Also, MUC16 overexpression induced cisplatin and gemcitabine resistance by downregulation of p53.Conclusions: Our findings indicate a significant role of MUC16 in tumorigenesis and metastasis of lung cancer cells possibly via regulation of TSPYL5 through the JAK2/STAT3/GR axis. Clin Cancer Res; 23(14); 3906-17. ©2017 AACR.
©2017 American Association for Cancer Research.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures




References
-
- Hollingsworth MA, Swanson BJ. Mucins in cancer: protection and control of the cell surface. Nat Rev Cancer. 2004;4(1):45–60. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

