Spontaneous activity in the piriform cortex extends the dynamic range of cortical odor coding
- PMID: 28196887
- PMCID: PMC5338513
- DOI: 10.1073/pnas.1620939114
Spontaneous activity in the piriform cortex extends the dynamic range of cortical odor coding
Abstract
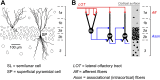
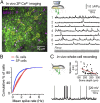
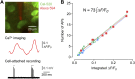
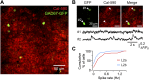
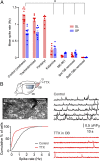
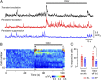
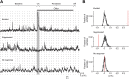
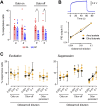
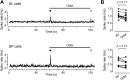
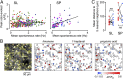
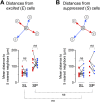
Neurons in the neocortex exhibit spontaneous spiking activity in the absence of external stimuli, but the origin and functions of this activity remain uncertain. Here, we show that spontaneous spiking is also prominent in a sensory paleocortex, the primary olfactory (piriform) cortex of mice. In the absence of applied odors, piriform neurons exhibit spontaneous firing at mean rates that vary systematically among neuronal classes. This activity requires the participation of NMDA receptors and is entirely driven by bottom-up spontaneous input from the olfactory bulb. Odor stimulation produces two types of spatially dispersed, odor-distinctive patterns of responses in piriform cortex layer 2 principal cells: Approximately 15% of cells are excited by odor, and another approximately 15% have their spontaneous activity suppressed. Our results show that, by allowing odor-evoked suppression as well as excitation, the responsiveness of piriform neurons is at least twofold less sparse than currently believed. Hence, by enabling bidirectional changes in spiking around an elevated baseline, spontaneous activity in the piriform cortex extends the dynamic range of odor representation and enriches the coding space for the representation of complex olfactory stimuli.
Keywords: anesthetic; calcium imaging; in vivo; olfaction; two-photon.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Task-Demand-Dependent Neural Representation of Odor Information in the Olfactory Bulb and Posterior Piriform Cortex.J Neurosci. 2019 Dec 11;39(50):10002-10018. doi: 10.1523/JNEUROSCI.1234-19.2019. Epub 2019 Oct 31. J Neurosci. 2019. PMID: 31672791 Free PMC article.
-
Population Coding in an Innately Relevant Olfactory Area.Neuron. 2017 Mar 8;93(5):1180-1197.e7. doi: 10.1016/j.neuron.2017.02.010. Epub 2017 Feb 28. Neuron. 2017. PMID: 28238549 Free PMC article.
-
Synaptic Organization of Anterior Olfactory Nucleus Inputs to Piriform Cortex.J Neurosci. 2020 Dec 2;40(49):9414-9425. doi: 10.1523/JNEUROSCI.0965-20.2020. Epub 2020 Oct 28. J Neurosci. 2020. PMID: 33115926 Free PMC article.
-
Coding odor identity and odor value in awake rodents.Prog Brain Res. 2014;208:205-22. doi: 10.1016/B978-0-444-63350-7.00008-5. Prog Brain Res. 2014. PMID: 24767484 Free PMC article. Review.
-
The Olfactory Mosaic: Bringing an Olfactory Network Together for Odor Perception.Perception. 2017 Mar-Apr;46(3-4):320-332. doi: 10.1177/0301006616663216. Epub 2016 Sep 29. Perception. 2017. PMID: 27687814 Free PMC article. Review.
Cited by
-
NMDA spikes mediate amplification of inputs in the rat piriform cortex.Elife. 2018 Dec 21;7:e38446. doi: 10.7554/eLife.38446. Elife. 2018. PMID: 30575520 Free PMC article.
-
Odor identity coding by distributed ensembles of neurons in the mouse olfactory cortex.Elife. 2017 May 10;6:e26337. doi: 10.7554/eLife.26337. Elife. 2017. PMID: 28489003 Free PMC article.
-
Combined Neural Tuning in Human Ventral Temporal Cortex Resolves the Perceptual Ambiguity of Morphed 2D Images.Cereb Cortex. 2020 Jul 30;30(9):4882-4898. doi: 10.1093/cercor/bhaa081. Cereb Cortex. 2020. PMID: 32372098 Free PMC article.
-
A Multicomponent Neuronal Response Encodes the Larval Decision to Pupariate upon Amino Acid Starvation.J Neurosci. 2018 Nov 21;38(47):10202-10219. doi: 10.1523/JNEUROSCI.1163-18.2018. Epub 2018 Oct 9. J Neurosci. 2018. PMID: 30301757 Free PMC article.
-
Semantic context-dependent neural representations of odors in the human piriform cortex revealed by 7T MRI.Hum Brain Mapp. 2024 Apr 15;45(6):e26681. doi: 10.1002/hbm.26681. Hum Brain Mapp. 2024. PMID: 38656060 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

