Modeling the two-way feedback between contractility and matrix realignment reveals a nonlinear mode of cancer cell invasion
- PMID: 28196892
- PMCID: PMC5338523
- DOI: 10.1073/pnas.1617037114
Modeling the two-way feedback between contractility and matrix realignment reveals a nonlinear mode of cancer cell invasion
Abstract
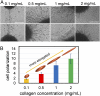
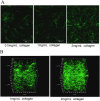
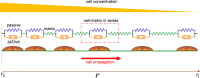
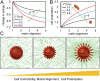
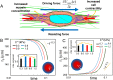
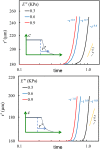
Cancer cell invasion from primary tumors is mediated by a complex interplay between cellular adhesions, actomyosin-driven contractility, and the physical characteristics of the extracellular matrix (ECM). Here, we incorporate a mechanochemical free-energy-based approach to elucidate how the two-way feedback loop between cell contractility (induced by the activity of chemomechanical interactions such as Ca2+ and Rho signaling pathways) and matrix fiber realignment and strain stiffening enables the cells to polarize and develop contractile forces to break free from the tumor spheroids and invade into the ECM. Interestingly, through this computational model, we are able to identify a critical stiffness that is required by the matrix to break intercellular adhesions and initiate cell invasion. Also, by considering the kinetics of the cell movement, our model predicts a biphasic invasiveness with respect to the stiffness of the matrix. These predictions are validated by analyzing the invasion of melanoma cells in collagen matrices of varying concentration. Our model also predicts a positive correlation between the elongated morphology of the invading cells and the alignment of fibers in the matrix, suggesting that cell polarization is directly proportional to the stiffness and alignment of the matrix. In contrast, cells in nonfibrous matrices are found to be rounded and not polarized, underscoring the key role played by the nonlinear mechanics of fibrous matrices. Importantly, our model shows that mechanical principles mediated by the contractility of the cells and the nonlinearity of the ECM behavior play a crucial role in determining the phenotype of the cell invasion.
Keywords: Rho pathway; cell contractility; cell invasion; fibrous matrices; matrix realignment.
Conflict of interest statement
The authors declare no conflict of interest.
Figures









References
-
- Paszek MJ, et al. Tensional homeostasis and the malignant phenotype. Cancer Cell. 2005;8(3):241–254. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

