Hyaluronidase 2 Deficiency Causes Increased Mesenchymal Cells, Congenital Heart Defects, and Heart Failure
- PMID: 28196902
- PMCID: PMC5331876
- DOI: 10.1161/CIRCGENETICS.116.001598
Hyaluronidase 2 Deficiency Causes Increased Mesenchymal Cells, Congenital Heart Defects, and Heart Failure
Abstract
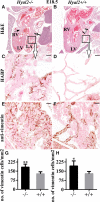
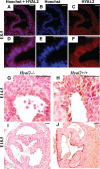
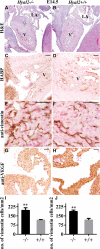
Background: Hyaluronan (HA) is required for endothelial-to-mesenchymal transition and normal heart development in the mouse. Heart abnormalities in hyaluronidase 2 (HYAL2)-deficient (Hyal2-/- ) mice and humans suggested removal of HA is also important for normal heart development. We have performed longitudinal studies of heart structure and function in Hyal2-/- mice to determine when, and how, HYAL2 deficiency leads to these abnormalities.
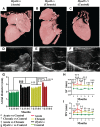
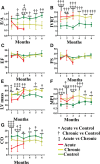
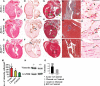
Methods and results: Echocardiography revealed atrial enlargement, atrial tissue masses, and valvular thickening at 4 weeks of age, as well as diastolic dysfunction that progressed with age, in Hyal2-/- mice. These abnormalities were associated with increased HA, vimentin-positive cells, and fibrosis in Hyal2-/- compared with control mice. Based on the severity of heart dysfunction, acute and chronic groups of Hyal2-/- mice that died at an average of 12 and 25 weeks respectively, were defined. Increased HA levels and mesenchymal cells, but not vascular endothelial growth factor in Hyal2-/- embryonic hearts, suggest that HYAL2 is important to inhibit endothelial-to-mesenchymal transition. Consistent with this, in wild-type embryos, HYAL2 and HA were readily detected, and HA levels decreased with age.
Conclusions: These data demonstrate that disruption of normal HA catabolism in Hyal2-/- mice causes increased HA, which may promote endothelial-to-mesenchymal transition and proliferation of mesenchymal cells. Excess endothelial-to-mesenchymal transition, resulting in increased mesenchymal cells, is the likely cause of morphological heart abnormalities in both humans and mice. In mice, these abnormalities result in progressive and severe diastolic dysfunction, culminating in heart failure.
Keywords: cor triatriatum; developmental biology; endocardium; extracellular matrix; live birth.
© 2016 The Authors.
Figures
Similar articles
-
Murine hyaluronidase 2 deficiency results in extracellular hyaluronan accumulation and severe cardiopulmonary dysfunction.J Biol Chem. 2013 Jan 4;288(1):520-8. doi: 10.1074/jbc.M112.393629. Epub 2012 Nov 21. J Biol Chem. 2013. PMID: 23172227 Free PMC article.
-
Hyaluronidase 2 (HYAL2) is expressed in endothelial cells, as well as some specialized epithelial cells, and is required for normal hyaluronan catabolism.Histochem Cell Biol. 2016 Jan;145(1):53-66. doi: 10.1007/s00418-015-1373-8. Epub 2015 Oct 29. Histochem Cell Biol. 2016. PMID: 26515055
-
Deletion of Fstl1 (Follistatin-Like 1) From the Endocardial/Endothelial Lineage Causes Mitral Valve Disease.Arterioscler Thromb Vasc Biol. 2017 Sep;37(9):e116-e130. doi: 10.1161/ATVBAHA.117.309089. Epub 2017 Jul 13. Arterioscler Thromb Vasc Biol. 2017. PMID: 28705792
-
Hyaluronidase 2 deficiency due to novel compound heterozygous variants in HYAL2: a case report of siblings with HYAL2 deficiency showing different clinical severity and literature review.J Hum Genet. 2025 Jun;70(6):321-324. doi: 10.1038/s10038-025-01333-1. Epub 2025 Mar 31. J Hum Genet. 2025. PMID: 40164710 Review.
-
Hyal2--less active, but more versatile?Matrix Biol. 2001 Dec;20(8):509-14. doi: 10.1016/s0945-053x(01)00170-6. Matrix Biol. 2001. PMID: 11731268 Review.
Cited by
-
Cardiac Molecular Analysis Reveals Aging-Associated Metabolic Alterations Promoting Glycosaminoglycans Accumulation via Hexosamine Biosynthetic Pathway.Adv Sci (Weinh). 2024 Oct;11(38):e2309211. doi: 10.1002/advs.202309211. Epub 2024 Aug 9. Adv Sci (Weinh). 2024. PMID: 39119859 Free PMC article.
-
Hyaluronan, a double-edged sword in kidney diseases.Pediatr Nephrol. 2022 Apr;37(4):735-744. doi: 10.1007/s00467-021-05113-9. Epub 2021 May 19. Pediatr Nephrol. 2022. PMID: 34009465 Free PMC article. Review.
-
Limiting extracellular matrix expansion in diet-induced obese mice reduces cardiac insulin resistance and prevents myocardial remodelling.Mol Metab. 2024 Aug;86:101970. doi: 10.1016/j.molmet.2024.101970. Epub 2024 Jun 20. Mol Metab. 2024. PMID: 38908792 Free PMC article.
-
Biomimetic Hyaluronan Binding Biomaterials to Capture the Complex Regulation of Hyaluronan in Tissue Development and Function.Biomimetics (Basel). 2024 Aug 17;9(8):499. doi: 10.3390/biomimetics9080499. Biomimetics (Basel). 2024. PMID: 39194478 Free PMC article. Review.
-
Hyaluronidase: structure, mechanism of action, diseases and therapeutic targets.Mol Biomed. 2025 Jul 12;6(1):50. doi: 10.1186/s43556-025-00299-y. Mol Biomed. 2025. PMID: 40646377 Free PMC article. Review.
References
-
- Pierpont ME, Basson CT, Benson DW, Jr, Gelb BD, Giglia TM, Goldmuntz E, et al. American Heart Association Congenital Cardiac Defects Committee, Council on Cardiovascular Disease in the Young. Genetic basis for congenital heart defects: current knowledge: a scientific statement from the American Heart Association Congenital Cardiac Defects Committee, Council on Cardiovascular Disease in the Young: endorsed by the American Academy of Pediatrics. Circulation. 2007;115:3015–3038. doi: 10.1161/CIRCULATIONAHA.106.183056. - PubMed
-
- Payne RM, Johnson MC, Grant JW, Strauss AW. Toward a molecular understanding of congenital heart disease. Circulation. 1995;91:494–504. - PubMed
-
- Camenisch TD, Spicer AP, Brehm-Gibson T, Biesterfeldt J, Augustine ML, Calabro A, Jr, et al. Disruption of hyaluronan synthase-2 abrogates normal cardiac morphogenesis and hyaluronan-mediated transformation of epithelium to mesenchyme. J Clin Invest. 2000;106:349–360. doi: 10.1172/JCI10272. - PMC - PubMed
-
- Chowdhury B, Xiang B, Muggenthaler M, Dolinsky VW, Triggs-Raine B. Hyaluronidase 2 deficiency is a molecular cause of cor triatriatum sinister in mice. Int J Cardiol. 2016;209:281–283. doi: 10.1016/j.ijcard.2016.02.072. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

